Pregnancy Tests: Clinical Applications and Tests for hCG

Pregnancy tests are designed to identify human chorionic gonadotropin (hCG) in either serum or urine. While the primary purpose of hCG testing is pregnancy detection, there are additional indications for measuring hCG, detailed in Box 1.
Human chorionic gonadotropin, a glycoprotein hormone originating from the placenta, circulates in maternal blood and is excreted intact through the kidneys. Comprising two polypeptide subunits—α (92 amino acids) and β (145 amino acids—hCG forms a non-covalent bond between these subunits. Structurally, hCG shares close similarities with three other glycoprotein hormones: luteinizing hormone (LH), follicle-stimulating hormone (FSH), and thyroid-stimulating hormone (TSH). The α subunits of hCG, LH, FSH, and TSH exhibit similarity, while the β subunits differ, conferring specific biological and immunological properties. Immunological tests deploy antibodies targeting the β-subunit of hCG to prevent cross-reactivity with LH, FSH, and TSH.
Box 1: Indications for measurement of β human chorionic gonadotropin
The syncytiotrophoblastic cells within the conceptus, and subsequently in the placenta, play a crucial role in the synthesis of hCG, also known as human chorionic gonadotropin. This hormone serves to support the corpus luteum of the ovary during the initial stages of pregnancy. The corpus luteum, in turn, produces progesterone, which acts to inhibit ovulation and thereby sustains the ongoing pregnancy.
As gestation progresses to the 7-10 week mark, the placenta becomes proficient in generating adequate quantities of progesterone independently. At this stage, the reliance on hCG diminishes, leading to a decline in its levels.
Clinical Applications of Tests for Human Chorionic Gonadotropin
- Early Pregnancy Diagnosis: The qualitative serum hCG test yields a positive result three weeks after the last menstrual period (LMP), while the urine hCG test shows positivity at five weeks post-LMP.
- Preventing Teratogenic Effects: Prior to prescribing specific medications such as oral contraceptives, steroids, and certain antibiotics, as well as before initiating radiological studies, radiotherapy, or chemotherapy, it is crucial to exclude pregnancy. This precautionary measure aims to prevent potential teratogenic effects on the developing fetus.
- Early Detection of Ectopic Pregnancy: Utilizing trans-vaginal ultrasonography (USG) and quantitatively estimating hCG proves valuable in the early identification of ectopic pregnancies, particularly before the occurrence of rupture.
- Assessment of Threatened Abortion: Serial quantitative estimation of hCG serves as a valuable tool in monitoring the progression of threatened abortion.
- Diagnosis and Monitoring of Gestational Trophoblastic Disease (GTD): The diagnostic and follow-up aspects of gestational trophoblastic disease (GTD) are effectively addressed through appropriate hCG assessments.
- Maternal Triple Test Screen: Conducted between the 14th and 19th weeks of gestation, the maternal triple test screen involves measuring hCG, α-fetoprotein, and unconjugated estriol in maternal serum. This screening method aids in identifying pregnant women at an elevated risk of Down syndrome and major congenital anomalies, including neural tube defects.
- Follow-Up of Germ Cell Tumors: The monitoring of ovarian or testicular germ cell tumors, known for hCG production, is facilitated through appropriate follow-up measures.
Normal Pregnancy
Box 2: Diagnosis of early pregnancy
- Positive serum hCG test: 8 days after conception or 3 weeks after last menstrual period (LMP)
- Positive urine hCG test: 21 days after conception or 5 weeks after LMP
- Ultrasonography for visualization of gestational sac:
- Transvaginal: 21 days after conception or 5 weeks after LMP
- Transabdominal: 28 days after conception or 6 weeks after LMP
In women with a regular menstrual cycle, the process of conception, marked by the fertilization of the ovum to form a zygote, typically takes place on day 14 within the fallopian tube. The resulting zygote then traverses the fallopian tube before reaching the uterus. Subsequent divisions of the zygote lead to the formation of a morula. At the 50-60-cell stage, this morula undergoes further development, giving rise to a blastocyst. Approximately 5 days post-fertilization, the blastocyst implants into the uterine wall. Trophoblastic cells, situated on the outer surface of the blastocyst, penetrate the endometrium and evolve into chorionic villi. Two primary types of trophoblasts emerge—the syncytiotrophoblast and cytotrophoblast. The development of the placenta originates from these chorionic villi. Once the placenta is formed, the term ‘embryo’ is applied to the conceptus. Subsequently, when the embryo progresses to the stage of developing most major organs, it is termed a ‘fetus’, a designation established after 10 weeks of gestation.
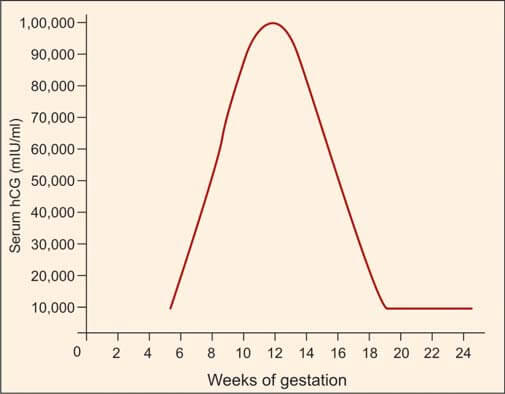
Human chorionic gonadotropin (hCG) is synthesized by syncytiotrophoblasts, primarily located in the placenta. Detectable amounts of hCG, approximately 5 mIU/ml, emerge in maternal serum around 8 days after conception, corresponding to 3 weeks after the last menstrual period (LMP). During the initial trimester, spanning the first 12 weeks from the onset of LMP, hCG levels undergo a rapid ascent, with a doubling time of roughly 2 days. The pinnacle, or peak level, is achieved between 8 and 10 weeks, reaching approximately 100,000 mIU/ml. Subsequently, a gradual decline ensues, stabilizing at 10,000-20,000 mIU/ml from 15-16 weeks onward, maintaining this level throughout the remainder of the pregnancy (refer to Figure 1). Post-delivery, hCG becomes non-detectable within about 2 weeks.
Referencing Box 2, the minimum time required for the earliest diagnosis of pregnancy is outlined, utilizing hCG tests and ultrasonography (USG).
There are two primary categories of pregnancy tests:
- Qualitative tests: These yield positive/negative results and are conducted on a urine sample.
- Quantitative tests: Providing numerical results, these tests are performed on either serum or urine. They find application in evaluating ectopic pregnancies, monitoring failing pregnancies, and conducting follow-up assessments for gestational trophoblastic disease.
Ectopic Pregnancy
Ectopic pregnancy denotes the implantation of a blastocyst at a site outside the uterine cavity. In over 95% of cases, this occurs in the fallopian tube. Timely identification and intervention in tubal ectopic pregnancies are imperative due to the associated risks of maternal mortality, stemming from rupture and hemorrhage, as well as the potential for future infertility. Ectopic pregnancy stands as a prominent cause of maternal death in the first trimester. Ultrasonography and the estimation of the β-subunit of human chorionic gonadotropin (hCG) are effective diagnostic tools in most cases.
The early diagnosis of an unruptured tubal pregnancy involves quantitative serum hCG estimation and ultrasonography. In a typical intrauterine pregnancy, the hCG titer doubles every 2 days until the first 40 days of gestation. An abnormally sluggish rise in hCG suggests a potentially nonviable pregnancy, encompassing ectopic or abnormal intrauterine pregnancies.
Transabdominal ultrasonography can identify the gestational sac in intrauterine pregnancies 6 weeks post-last menstrual period (LMP), concomitant with a serum hCG level exceeding 6500 mIU/ml. The absence of a visualized gestational sac at this hCG level raises suspicions of an ectopic pregnancy. Transvaginal ultrasonography, more sensitive than its abdominal counterpart, can detect an ectopic pregnancy approximately 1 week earlier and identifies a gestational sac with a β-hCG level of 1000-1500 mIU/ml. Consequently, the absence of a visualized gestational sac at a β-hCG level exceeding 1500 mIU/ml raises concerns for ectopic pregnancy.
Early diagnosis of ectopic pregnancies allows for the administration of intramuscular methotrexate instead of surgery, leading to the dissolution of the conceptus and enhancing the prospects of future fertility. Serial hCG measurements post-surgical removal aid in detecting the persistence of trophoblastic tissue.
Abortion
The termination of a pregnancy prior to the viability of the fetus (typically before 20 weeks) is termed abortion.
In cases of threatened abortion, there is the presence of vaginal bleeding, yet the internal os remains closed, and the abortion process, though initiated, is still reversible. There exists the possibility that the pregnancy may persevere.
Diagnosis and management of abortion benefit from the utilization of serial quantitative titers of human chorionic gonadotropin (hCG), revealing a lack of the anticipated doubling of hCG levels, along with ultrasonography (USG). These combined approaches contribute to a comprehensive understanding and effective handling of cases involving abortion.
Gestational Trophoblastic Disease (GTD)
This condition is marked by the proliferation of trophoblastic tissue associated with pregnancy. The primary forms of gestational trophoblastic disease (GTD) include hydatidiform (vesicular) mole, which is benign, and choriocarcinoma, which is malignant. The clinical features of GTD encompass:
- A brief history of amenorrhea followed by vaginal bleeding.
- Enlargement of the uterus beyond the corresponding gestational age, characterized by a soft and doughy consistency upon palpation, devoid of fetal parts and fetal heart sounds.
- Excessive nausea and vomiting attributed to elevated human chorionic gonadotropin (hCG) levels.
- The distinctive snowstorm appearance on pelvic ultrasonography (USG).
Quantitative estimation of hCG is useful in both the diagnosis and management of GTD.
Trophoblastic cells in GTD exhibit heightened hCG production compared to those in a normal pregnancy at the equivalent gestational age. The concentration of hCG mirrors the tumor load, and unlike normal pregnancies that reach a plateau by the end of the first trimester, hCG levels in GTD continue to rise beyond 10 weeks of gestation.
Following uterine evacuation, the recommended practice involves weekly hCG estimations until three consecutive results, obtained weekly, return negative. In cases of vesicular mole evacuation, hCG becomes undetectable in approximately 80% of cases within 2-3 months of follow-up. Persistent or rising hCG levels suggest ongoing GTD, prompting the initiation of chemotherapy.
Post-therapy, negative hCG results should undergo regular follow-up every 3 months for 1-2 years to monitor the absence of recurrence. This meticulous monitoring approach ensures comprehensive management and surveillance in cases of GTD.
Laboratory Tests for Human Chorionic Gonadotropin
These are classified into two main groups:
- Biological assays or bioassays
- Immunological assays
Bioassays
In bioassay, the impact of hCG is evaluated using laboratory animals in controlled settings. However, bioassays present several drawbacks, including the necessity for animal facilities, the requirement for animal standardization, prolonged test result timelines, limited sensitivity, and high associated costs. Consequently, immunological assays have emerged as a preferred alternative, supplanting bioassays.
The Ascheim-Zondek test involves the injection of urine from a pregnant woman into immature female mice. A positive outcome is indicated by the development of hemorrhagic corpora lutea in the ovaries after a 4-day period. The Friedman test follows a similar approach, with urine injection, but employs female rabbits instead. In the rapid rat test, the injection of urine containing hCG into female rats induces hyperemia and hemorrhaging in the ovaries. Another distinctive test involves the observation of spermatozoa release from male frogs subsequent to the injection of urine containing hCG.
Immunological Assays
These tests offer rapid and highly sensitive means for detecting and quantifying hCG levels. Variable outcomes arise from distinct immunological tests using the same serum sample, attributed to variations in the specificity of immunoassays for complete hCG, β-subunit, and β-core fragment. A range of commercially available immunological tests employs diverse principles, including agglutination inhibition assay, enzyme immunoassay (such as ELISA), radioimmunoassay (RIA), and immunoradiometric assay.
Among the commonly utilized qualitative urine tests is the agglutination inhibition assay. For optimal results, an early morning urine specimen is preferred due to its elevated hCG concentration. Factors contributing to false-positive results encompass red cells, leukocytes, bacteria, certain drugs, proteins, and an excess of luteinizing hormone (present in menopause or midcycle LH surge) in the urine. Some individuals exhibit anti-mouse antibodies employed in the test, while others harbor hCG-like substances in circulation, leading to false-positive outcomes. These anti-mouse antibodies, known as ‘heterophil’ antibodies, also pose interference in other antibody-based tests. Causes of false-negative results encompass fetal death, abortion, dilute urine, and the low sensitivity inherent in a specific test. Renal failure can result in the accumulation of interfering substances, introducing inaccuracies in test outcomes.
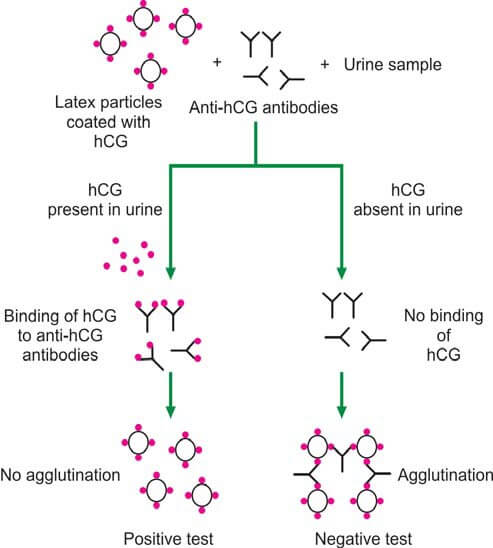
In the latex particle agglutination inhibition test (depicted in Figure 2), patient urine is incubated with anti-hCG antibodies, followed by the addition of hCG-coated latex particles. If hCG is present in the urine, anti-hCG serum is neutralized, preventing latex particle agglutination (yielding a positive test). Conversely, the absence of hCG in urine results in latex particle agglutination (indicating a negative test). This method is commonly implemented as a slide test, offering rapid results within a few minutes.
The agglutination inhibition test boasts a sensitivity exceeding 200 units/liter of hCG. However, radioimmunoassay, enzyme immunoassay, and radioimmunometric assay are recognized for surpassing the agglutination inhibition assay in terms of sensitivity and reliability.
Quantitative tests are useful in detecting very early pregnancy, estimating gestational age, diagnosing ectopic pregnancy, assessing threatened abortion, and managing gestational trophoblastic disease (GTD).
Reference Ranges
- Serum human chorionic gonadotropin:
- Non-pregnant females: <5.0 mIU/ml
- Pregnancy:
- 4 weeks after LMP: 5-100 mIU/ml
- 5 weeks after LMP: 200-3000 mIU/ml
- 6 weeks after LMP: 10,000-80,000 mIU/ml
- 7-14 weeks: 90,000-500,000 mIU/ml
- 15-26 weeks: 5000-80000 mIU/ml
- 27-40 weks: 3000-15000 mIU/ml
Further Reading: Semen Analysis for Investigation of Infertility
- Comment
- Posted by Dayyal Dg.
- Clinical Pathology
- Pathology
- Pathology Notes
- Notes
- Article
- How to
- Laboratory
- Laboratory Technique
- Laboratory Test Procedure
- Pregnancy Tests Procedures
- Pregnancy Test Instructions
- Pregnancy Testing Method
- Common Testing During Pregnancy
- Procedure for Pregnancy Testing
- Pregnancy Tests and Procedures
- When to Take Pregnancy Tests
- Diseases and Disorders
- Lab Test