Displaying items by tag: Hemotological Tests
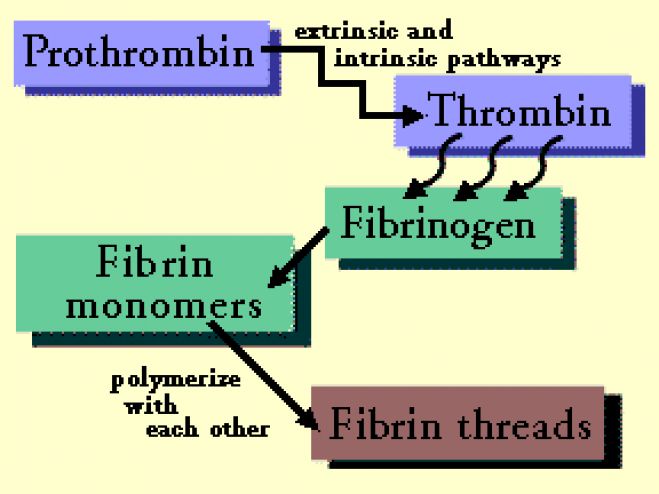
Thrombin Time (TT)
Thrombin is added to patient’s plasma and time required for clot formation is noted.
Thrombin solution.
Venous blood is collected from antecubital fossa with a plastic, siliconized glass, or polypropylene syringe and a large bore needle (20 or 21 G in adults, 22 or 23 G in infants). Blood should never be collected from indwelling intravenous lines, as these often contain heparin. Glass syringe should not be used for collection since it activates coagulation. The blood is drawn gently but quickly after a single, smooth venepuncture. The needle is detached from the syringe, and the sample is passed gently into the plastic container. After capping the container, the blood and the anticoagulant are mixed immediately by gentle inversion 5 times. The anticoagulant used for coagulation studies is trisodium citrate (3.2%), with anticoagulant to blood proportion being 1:9. Most coagulation studies require platelet poor plasma (PPP). To obtain PPP, blood sample is centrifuged at 3000-4000 revolutions per minute for 15-30 minutes. Coagulation studies are carried out within 2 hours of collection of sample.
Take 0.1 ml of buffered saline in a test tube and add 0.1 ml of plasma. Note clotting time after addition of 0.1 ml of thrombin solution.
± 3 seconds of control.
(1) Disorders of fibrinogen: Prolongation of TT occurs in afibrinogenemia (virtual absence of fibrinogen), hypofibrinogenemia (fibrinogen less than 100 mgs/dl), and dysfibrinogenemia (dysfunctional fibrinogen).
(2) Heparin therapy: Heparin inhibits action of thrombin.
(3) Presence of fibrin degradation products (FDPs): These interfere with fibrin monomer polymeri-zation. TT is repeated using a mixture of normal plasma and patient’s plasma. If TT remains prolonged, then FDPs are present (provided patient is not receiving heparin).
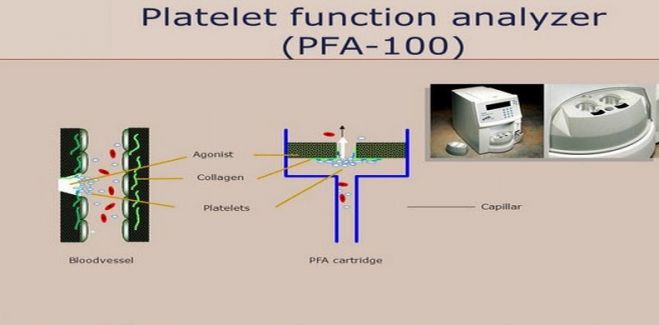
Platelet Function Analyzer (PFA-100)
This is a newly introduced screening test for platelet function that assesses both platelet adhesion and aggregation. This method uses an instrument called as PFA-100 in which anticoagulated whole blood is passed at a high shear rate through small membranes that have been coated with either collagen and epinephrine or collagen and ADP.
Laboratory Tests in Bleeding Disorders
Bleeding disorders are the result of a generalized defect in hemostasis due to abnormalities of blood vessels, platelets, or coagulation factors.
Initial tests, which should be performed in a suspected bleeding disorder, are complete blood count including blood smear, platelet count, bleeding time, clotting time, prothrombin time, and activated partial thromboplastin time. Depending on the results of these screening tests, one or more specific tests are carried out for definitive diagnosis (e.g. platelet function studies, assays of
coagulation factors, and test for fibrin degradation products). Abnormalities of blood vessels are usually not detectable by laboratory tests for hemostasis, and their diagnosis requires correlation of clinical and other investigations.
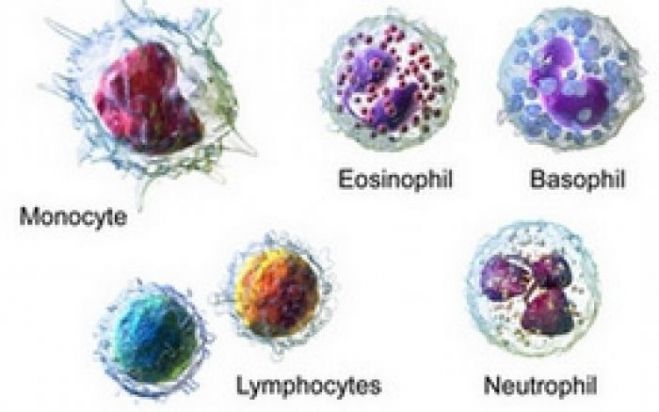
A complete blood count and a blood smear can provide information in the form of:
• Presence of cytopenia (anemia, leukopenia, thrombocytopenia)
• Red cell abnormalities (especially fragmented red cells which may indicate disseminated intravascular
coagulation)
• White cell abnormalities (like abnormal cells in leukemias)
• Abnormalities of platelets: thrombocytopenia (normally there is 1 platelets per 500-1000 red cells), giant platelets (seen in myeloproliferative disorders and Bernard-Soulier syndrome), and isolated discrete platelets without clumping in finger-prick smear (seen in uremia, Glanzmann’s thrombasthenia).
(2) Platelet Count
(3) Bleeding Time (BT)
(4) Clotting Time (CT)
(5) Prothrombin Time (PT)
(6) Activated Partial Thromboplastin Time (APTT)
(7) Thrombin Time (TT)
(8) Platelet Function Analyzer-100
| LICENSE: This article uses material from the Wikipedia article "HEMOSTASIS", which is released under the Creative Commons Attribution-Share-Alike License 3.0. |
Life Cycle of Malaria Parasite
Life history of malaria parasite consists of two cycles of development: asexual cycle or schizogony that occurs in humans and sexual cycle or sporogony that occurs in mosquitoes.
Asexual cycle (human cycle, schizogony)
This occurs in the liver cells and red blood cells of infected humans, and therefore humans are the intermediate hosts of the malaria parasite (Schizogony refers to the process of reproduction in protozoa in which there is production of daughter cells by fission). The human cycle begins when infected female Anopheles mosquito bites a person and sporozoites are injected into the circulation. There are four stages of human cycle.
(a) Pre-erythrocytic schizogony (Hepatic schizogony):
Inoculated sporozoites rapidly leave the circulation to enter the liver cells where they develop into hepatic (pre-erythrocytic) schizonts (Schizonts are cells undergoing schizogony). One sporozoite produces one tissue form. Hepatic schizonts rupture to release numerous merozoites in circulation (Merozoites are daughter cells produced after schizogony). Up to 40,000 merozoites are produced in the hepatic schizont.
In P. falciparum infection, all of the hepatic schizonts mature and rupture simultaneously; dormant forms do not persist in hepatocytes. In contrast, some of the sporozoites of P. vivax and P. ovale remain dormant after entering liver cells and develop into schizonts after some delay. Such persistent forms are called as hypnozoites; they develop into schizonts at a later date and are a cause of relapse.
(b) Erythrocytic schizogony:
Merozoites released from rupture of hepatic schizonts enter the red blood cells via specific surface receptors. These merozoites become trophozoites that utilize red cell contents for their metabolism. A brown-black granular pigment (malaria pigment or hemozoin) is produced due to breakdown of hemoglobin by malaria parasites. The fully formed trophozoite develops into a schizont by multiple nuclear and cytoplasmic divisions. Mature schizonts rupture to release merozoites, red cell contents, malarial toxins, and malarial pigment. (This pigment is taken up by monocytes in peripheral blood and by macrophages of reticulo-endothelial system. In severe cases, organs which are rich in macrophages like spleen, liver, lymph nodes, and bone marrow become slate-gray or black in color due to hemozoin pigment). Rupture of red cell schizonts corresponds with clinical attack of malaria. Released merozoites infect new red cells and enter another erythrocytic schizogony cycle. This leads to rapid amplification of plasmodia in the red cells of the human host. In P. falciparum, P. vivax, and P. ovale infections, cycle of schizogony lasts for 48 hours, while in P. malarie infection it lasts for 72 hours. Merozoites of P. vivax and P. ovale preferentially invade young red cells or reticulocytes while those of P. falciparum infect red cells of all ages. Senescent red cells are preferred by P. malariae.
P. vivax, P. ovale, and P. malariae complete the erythrocyte schizogony in general circulation. Schizonts of P. falciparum induce membrane changes in red cells, which causes them to adhere to the capillary endothelial cells (cytoadherence). Therefore, in P. falciparum infection, erythrocyte schizogony is completed in capillaries of internal organs and usually only ring forms are seen in circulation.
(c) Gametogony:
After several cycles of erythrocytic schizogony, some merozoites, instead of developing into trophozoites and schizonts, transform into male and female gametocytes. These sexual forms are infective to mosquito and the person harboring them is called as a “carrier”. Gametocytes are not pathogenic for humans.
(d) Exoerythrocytic schizogony:
In P. vivax and P. ovale infections, some of the sporozoites in liver cells persist and remain dormant. These dormant forms in liver cells are called as hypnozoites. They become active and develop into schizonts a few days, months, or even years later. These schizonts rupture, release merozoites, and cause relapse. Exoerythrocytic schizogony is absent in P. falciparum infection and therefore relapse does not occur. Hence, P. vivax and P. ovale are called as relapsing plasmodia while P. falciparum and P. malariae are known as non-relapsing plasmodia.
Sexual cycle (mosquito cycle, sporogony)
The sexual cycle begins when a female Anopheles mosquito ingests mature male and female gametocytes during a blood meal. First, 4-8 microgametes are produced from one male gametocyte (microgametocyte) in the stomach of the mosquito; this is called as exflagellation. The female gametocyte (macrogametocyte) undergoes maturation to produce one macrogamete. By chemotaxis, microgametes are attracted toward the macrogamete; one of the microgametes fertilizes the macrogamete to produce a zygote. The zygote becomes motile and is called as ookinete. Ookinete penetrates the lining of the stomach and comes to lie on the outer surface of the stomach where it develops into an oocyst. On further growth and maturation, multiple sporozoites are formed within the oocyst. After complete maturation, oocyst ruptures to release sporozoites into the body cavity of the mosquito. Most of the sporozoites migrate to the salivary glands. Infection is transmitted to the humans by the bite of the mosquito through saliva when it takes a blood meal.
How to Count Reticulocytes (Manual Method)
Reticulocytes
Reticulocytes are young or juvenile red cells released from the bone marrow into the bloodstream and that contain remnants of ribonucleic acid (RNA) and ribosomes but no nucleus. After staining with a supravital dye such as new methylene blue, RNA appears as blue precipitating granules or filaments within the red cells. Following supravital staining, any nonnucleated red cell containing 2 or more granules of bluestained material is considered as a reticulocyte (The College of American Pathology). Supravital staining refers to staining of cells in a living state before they are killed by fixation or drying or with passage of time. Reticulocyte count is performed by manual method.
PRINCIPLE
A few drops of blood (collected in EDTA) are incubated with new methylene blue solution which stains granules of RNA in red cells. A thin smear is prepared on a glass slide from the mixture and reticulocytes are counted under the microscope. Number of reticulocytes is expressed as a percentage of red cells.
REAGENT
New methylene blue solution is prepared as follows:
- New methylene blue: 1.0 gm
- Sodium citrate: 0.6 gm
- Sodium chloride: 0.7 gm
- Distilled water: 100 ml
Reagent should be kept stored in a refrigerator at 2-6°C and filtered before use.
Suitable alternatives to new methylene blue are brilliant cresyl blue and azure B.
SPECIMEN
Capillary blood or EDTA anticoagulated venous blood can be used.
METHOD
(1) Take 2-3 drops of filtered new methylene blue solution in a 12 × 75 mm test tube.
(2) Add equal amount of blood and mix well.
(3) Keep the mixture at room temperature or at 37°C for 15 minutes.
(4) After gentle mixing, place a small drop from the mixture on a glass slide, prepare a thin smear, and allow to dry in the air.
(5) Examine under the microscope using oil-immersion objective. Mature red cells stain pale green blue. Reticulocytes show deep blue precipitates of fine granules and filaments in the form of a network (reticulum). Most immature reticulocytes show a large amount of precipitated material in the form of a mass, while the most mature reticulocytes show only a few granules or strands. Any nonnucleated red cell is considered as a reticulocyte if it contains 2 or more blue-stained particles of ribosomal RNA.
(6) Count 1000 red cells and note the number of red cells that are reticulocytes. Counting error is minimized if size of the microscopic field is reduced. This is achieved by using a Miller ocular disk inserted in the eyepiece; it divides the field into two squares (one nine times larger in size than the other). Reticulocytes are counted in both the squares and the red cells are counted in the smaller square.
REPORTING THE RESULT
(1) Reticulocyte percentage: The most common method of reporting is reticulocyte percentage which is calculated from the following formula:
NRBC
Where NR is the Number of reticulocyte counted and NRBC is number of red blood cell counted.
Reference range is 0.5%-2.5% in adults and children. Reticulocyte count is higher in newborns.
(2) Absolute reticulocyte count = Reticulocyte percentage × Red cell count
Normal: 50,000 to 85,000/cmm
(3) Corrected reticulocyte count (Reticulocyte index)
Normal PCV
Corrected reticulocyte count > 2% indicates reticulocyte release appropriate for the degree of anemia. If < 2%, reticulocyte release is inappropriate.
(4) Reticulocyte maturation production index
Estimated maturation time in days
REFERENCE RANGES
- Reticulocyte percentage: 0.5 2.5%
- Absolute reticulocyte count: 50,000-85,000/cmm
Reticulocyte
USES
- As one of the baseline studies in anemia with no obvious cause
- To diagnose anemia due to ineffective erythropoiesis (premature destruction of red cell precursors in bone marrow seen in megaloblastic anemia and thalassemia) or due to decreased production of red cells: In hypoplastic anemia or in ineffective erythropoiesis, reticulocyte count is low as compared to the degree of anemia. Increased erythropoiesis (e.g. in hemolytic anemia, blood loss, or specific treatment of nutritional anemia) is associated with increased reticulocyte count. Thus reticulocyte count is used to differentiate hypoproliferative anemia from hyperproliferative anemia.
- To assess response to specific therapy in iron deficiency and megaloblastic anemias.
- To assess response to erythropoietin therapy in anemia of chronic renal failure.
- To follow the course of bone marrow transplantation for engraftment
- To assess recovery from myelosuppressive therapy
- To assess anemia in neonate
Reticulocyte Count Test
Reticulocyte Values In Sickle Cell Anemia
Reticulocyte Count Reference Range
Supravital Stain Reticulocytes
Romanowsky Stain
Reticulocyte Count Method
Reticulocyte Staining Procedure
Reticulocytes With Supravital Stain
Reticulocyte NRBC
Miller Disc Retic Count
Reticulocyte Count Procedure Miller Disc
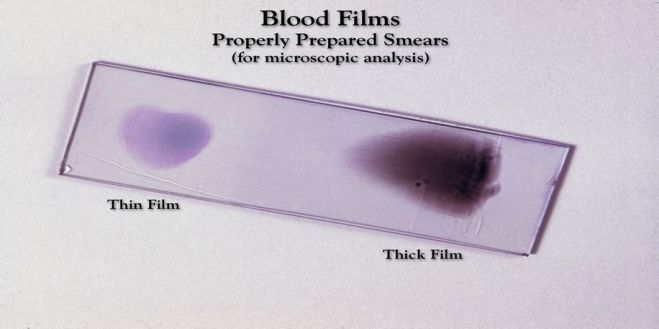
PREPARATION OF BLOOD SMEAR (WEDGE METHOD)
(2) A 'spreader' slide is placed at an angle of 30° in front of the drop and then drawn back to touch the drop of blood. Blood spreads across the line of contact of two slides.
(3) Smear is made by smooth, forward movement of the 'spreader' along the slide. The whole drop should be used up 1 cm before the end of the slide. The length of the smear should be about 3 cm. The 'spreader' should not be raised above the slide surface till whole drop of blood is spread out.
(5) Patient's name or laboratory number and date are written (with a lead pencil, a permanent marker pen, or a diamond pencil) on the thicker end of the smear.
(6) The smear is fixed immediately with absolute methyl alcohol (which should be moisture- and acetone-free) for 2-3 minutes in a covered jar (Absolute ethyl alcohol can also be used, but not methylated spirit as it contains water). Aim of fixation is to prevent washing off of the smear from the slide. Following this, color of the smear becomes light brown. This fixation is desirable even when Leishman stain is used which contains methyl alcohol. This is because Leishman stain may have absorbed moisture leading to poor fixation. If methanol is contaminated with water, sharpness of cell morphology is lost and there is vacuolation of red cells. Methanol should be acetone-free since acetone washes out nuclear stain. (In many laboratories, slide is stained immediately after air-drying without prior fixation, and the results are satisfactory; however, if delay of >4 hours is anticipated between air-drying and staining, the slide should be fixed. If not, a background gray-blue staining of plasma occurs).
(1) Making a 'spreader' slide—a glass slide with absolutely smooth edges should be selected and one or both corners at one end of the slide should be broken off. The 'spreader' slide should be narrower (width of about 15 mm) so that edges of the smear can be examined microscopically. The 'spreader' slide should be discarded after use. If the same is to be reused, its edge should be thoroughly cleaned and dried (otherwise carryover of cells or parasites can occur).
(2) A well-spread blood smear (a) is tongue-shaped with a smooth tail, (b) does not cover the entire area of the slide, (c) has both thick and thin areas with gradual transition, and (d) does not contain any lines or holes.
(3) By changing the angle of the 'spreader' and its speed, thickness of the blood smear can be controlled. In patients with anemia, a thicker smear can be obtained by increasing the angle and the speed of spreading. In patients with polycythemia, a thinner smear is obtained by decreasing the 'spreader' angle and the speed of spreading.
(5) It is recommended to stain blood films in reagent filled Coplin jars (rather than covering them with the staining solution) to avoid formation of stain precipitates due to evaporation.
(6) A drawback of this method is uneven distribution of leukocytes (i.e. monocytes, neutrophils, and abnormal cells are pushed towards the extreme tail end of the smear) and distortion of red cell morphology at the edges.
(7) Blood smear is covered with a coverslip and mounted in a mounting medium (e.g. DPX) for protection against mechanical damage and deterioration of staining with time on exposure to air.
STAINING OF BLOOD SMEAR
Blood smears are routinely stained by one of the Romanowsky stains. Romanowsky stains consist of a combination of acidic and basic dyes and after staining various intermediate shades are obtained between the two polar (red and blue) stains. Romanowsky stains include May-Grunwald-Giemsa, Jenner, Wright's, Leishman's, and Field's stains. Staining properties of the Romanowsky stains are dependent on two synthetic dyes: methylene blue and eosin. International Committee for Standardization in Haematology has recommended a highly purified standardized stain, which contains azure B and eosin Y; it, however, is very expensive. Romanowsky stains are insoluble in water but soluble in methyl alcohol. Methyl alcohol acts as a solvent as well as a fixative. Staining reaction is pH-dependent. These stains have a tendency towards precipitation and should be filtered before use.
Methylene blue and azure B are basic (cationic) dyes and have affinity for acidic components of the cells (like nucleic acids or basophil granules) and impart purpleviolet color to the nuclear chromatin, dark blue-violet color to the basophil granules, and deep blue color to the cytoplasm of lymphocytes. Eosin is an acidic (anionic) dye and has affinity for basic components like hemoglobin (stained pink-red), and granules in eosinophils (stained orange-red). Neutrophil granules are slightly basic and stain violet-pink or lilac.
Romanowsky stains impart more colours than just blue (from methylene blue or azure B) and red-orange (from eosin Y). Usefulness of the Romanowsky stains lies in their ability to differentially stain leucocyte granules.
Method of Leishman staining is given below:
Reagents
(1) Leishman stain: William Boog Leishman, a British pathologist, modified the original Romanowsky method and devised a stain which is widely known as Leishman's stain. This consists of methylene blue and eosin dissolved in absolute methyl alcohol. Commercially available Leishman stain powder (0.6 gram) is mixed with water-free absolute methyl alcohol (400 ml). Prepared stain should be kept tightly stoppered in a brown bottle and stored in a cool, dark place at room temperature. Exposure to direct sunlight causes deterioration of the stain. After preparation, stain should be kept for 3-5 days before using since it improves the quality of the stain.
(2) Buffered water (pH 6.8).
Method
(1) Air-dry the smear and fix with methanol for 2-3 minutes.
(2) Cover the smear with Leishman stain for 2 minutes.
(3) After 2 minutes, add twice the volume of buffered water and leave for 5-7 minutes. A scum of metallic sheen forms on the surface.
(4) Wash the stain away in a stream of buffered water. Tap water can also be used for washing if it is not highly alkaline or highly acid.
(5) Wipe the back of the slide clean and set it upright in the draining rack to dry.
(6) Mount the slide in a suitable mounting medium (e.g. DPX) with a clean and dry 25 × 25 mm coverslip.
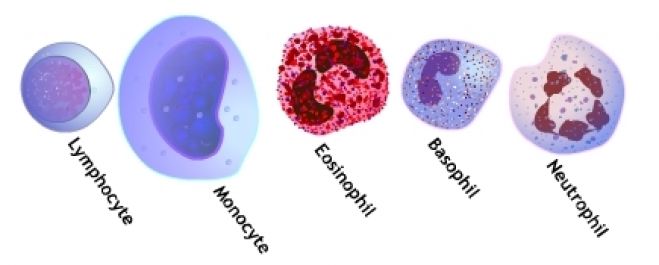
• Red cells: pink-red or deep pink
• Polychromatic cells (Reticulocyt-es): Gray-blue
• Neutrophils: Pale pink cytopla-sm; mauve-purple granules
• Eosinophils: Pale-pink cytoplasm; orange-red granules
• Basophils: Blue cytoplasm; dark blue-violet granules
• Monocytes: Gray-blue cytoplasm; fine reddish (azurophil) granules
• Small lymphocytes: Dark blue cy-toplasm
• Platelets: Purple
• Nuclei of all cells: Purple-violet
Blood Smear
Blood films are made by placing a drop of blood on one end of a slide, and using a spreader slide to disperse the blood over the slide's length. The aim is to get a region, called a monolayer, where the cells are spaced far enough apart to be counted and differentiated. The monolayer is found in the "feathered edge" created by the spreader slide as it draws the blood forward.
The slide is left to air dry, after which the blood is fixed to the slide by immersing it briefly in methanol. The fixative is essential for good staining and presentation of cellular detail. After fixation, the slide is stained to distinguish the cells from each other.
Routine analysis of blood in medical laboratories is usually performed on blood films stained with Romanowsky, Wright's, or Giemsa stain. Wright-Giemsa combination stain is also a popular choice. These stains allow for the detection of white blood cell, red blood cell, and platelet abnormalities. Hematopathologists often use other specialized stains to aid in the differential diagnosis of blood disorders.
After staining, the monolayer is viewed under a microscope using magnification up to 1000x. Individual cells are examined and their morphology is characterized and recorded.
(2) To monitor the effect of chemotherapy and radiotherapy on bone marrow.
(3) To provide direction for further investigations that will help in arriving at the correct diagnosis (e.g. in infections, drug toxicity, etc.). Blood smear examination is therefore indicated in clinically suspected cases of anemia, thrombocytopenia, hematological malignancies (leukemia, lymphoma, multiple myeloma), disseminated intravascular coagulation, parasitic infections (like malaria or filaria), infectious mononucleosis, and various inflammatory, or malignant diseases.
Erythrocyte Sedimentation Rate (ESR): Significance, Stages, Methods, Interpretation and Reference Ranges
The erythrocyte sedimentation rate (ESR) measures the rate of settling (sedimentation) of erythrocytes in anticoagulated whole blood. Anticoagulated blood is allowed to stand in a glass tube for 1 hour and the length of column of plasma above the red cells is measured in millimeters; this corresponds to ESR. There are four different methods for the estimation of ESR.
WHO Hemoglobin Color Scale for the Estimation of Hemoglobin
This method conceived and formulated by Stott and Lewis in 1995. This method is much similar in principle to the now outdated Tallqvist method. Positive technical changes have been made to improve the validity, accuracy and reliability. This method is simple, swift, reliable and inexpensive. This method is reliable and trustworthy within 1 gram/dl for diagnosis of anemia. The World Health Organization (WHO) Hemoglobin Color Scale consists of a printed set of colors corresponding to the hemoglobin value from 4 to 14 grams/dl. On a strip of chromatography paper, a drop of blood is placed and then the developed color is matched visually against the printed color scale. Research has proven that performance is greater than 90% in detecting anemia and 86% in classifying the grade of anemia. The World Health Organization (WHO) has developed hemoglobin color scale after extensive and vast field trails. It is mainly planned for the detection, treatment and control of anemia in under-resourced countries. It is especially use for the screening of blood donors, for screening women and children in health scheme, examine iron therapy, selection-making concerning referral to a hospital, and as a point of care tool.
Difference Between Total Leukocyte Count and Differential Leukocyte Count!
Variations of leukocyte count occur in many infectious, hematologic, inflammatory, and neoplastic diseases.
Therefore, laboratory evaluation of almost all patients begins with the examination of the patient’s blood for total leukocyte count and examination of the peripheral blood smear for the differential leukocyte count as well as blood cells picture. Usually, some clinical interpretations may be made from the total leukocyte count and differential leukocyte count.
From total leukocyte count and differential leukocyte count, the absolute count of each leukocyte type can be calculated. The absolute leukocyte count provides a more accurate picture than the differential leukocyte count. (For example, a chronic lymphocytic leukemia patient has a total leukocyte count of 100 x 103 cells µI and a differential leukocyte count of 7 percent neutrophils and 90 percent lymphocytes. By looking at the differential leukocyte count of 7 percent neutrophils alone one get the impression of very low neutrophils in this patient But if the absolute count of neutrophils is calculated, one will be surprised to see a normal neutrophil number in this patient.
Absolute neutrophil count = Total leukocyte count x neutrophil percent
=100 x 103 x 7/100 = 7 x 103 = 7000 / µl
At times of acute bacterial infection enormous numbers of neutrophils are required. Therefore, large number of neutrophils is released from bone marrow to cope with this requirement. Consequently, the number as well as the percentage of neutrophils in the blood increases several fold. Hence, an increase in total leukocyte count with increase in percentage of neutrophil is taken as an important indication of acute bacterial infection.
Leukocytosis
The terms leukocytosis and leukopenia indicate increase or decrease in the total number of leukocytes, respectively. Increase in numbers of neutrophils, eosinophils, lymphocytes, and monocytes are known as neutrophilia, eosinophilia, lymphocytosis, and monocytosis respectively.
i. Neutrophilia is the increase in peripheral blood absolute neutrophil count, above the upper limit of normal of 7.5 x 109/L (in adults). Bacterial infection is one of the common causes of neutrophilia. Neutrophilia is not a feature of viral infections (However, the development of neutrophila late in the course of a viral infection may indicate the emergence of secondary bacterial infection). There is a storage pool of mature neutrophils in the bone marrow (Such storage pool does not occur for other leukocytes).
In response to stress, such as infections the neutrophils from storage pool are released into circulation, resulting in a rise in total leukocyte count and neutrophil percentage. Moreover, there is increased neutrophil production in the bone marrow. Increased neutrophil production during bacterial infection is usually associated with the entry of less mature neutrophils from bone marrow into blood. This is indicated by the appearance of neutrophils with lesser number of nuclear segmentation in the peripheral blood picture. Band cells may also be seen in the peripheral blood smear.
This is referred to as a ‘shift to the left’. The leukemoid reaction is a reactive and excessive leukocytosis, wherein the peripheral blood smear shows the presence of immature cells (e.g. myeloblasts, promyelocytes, and myelocytes). Leukemoid picture occurs in severe or chronic infections and hemolysis. Another most common change that occurs in neutrophils during infection is the presence of toxic basophilic inclusions in the cytoplasm.
Eosinophilia is an increase in peripheral blood absolute eosinophil count beyond 0.4 x 109/L (in adults). Eosinophilia is usually associated with allergic conditions such as asthma and hay fever and parasitic infections. Eosinophilia may also occur in reactions to drugs.
Leukopenia
Decrease in total leukocyte count is known as leukopenia. Reduction in the number of neutrophils is the most frequent cause of leukopenia.
i. Neutropenia is the decrease in peripheral blood absolute neutrophil count below the lower limit of normal of 2 x 109/L (in adults). Neutropenic patients are more vulnerable to infection.
ii. Lymphopenia in adults is the decrease in peripheral blood absolute lymphocyte count below the lower limit of normal of 1.5 x 109/L. Lymphopenia is common in the leukopenic prodromal phase of many viral infections. A selective depletion of helper T lymphocytes (CD4+) with or without absolute lymphopenia occurs in acquired immunodeficiency disease (AIDS).
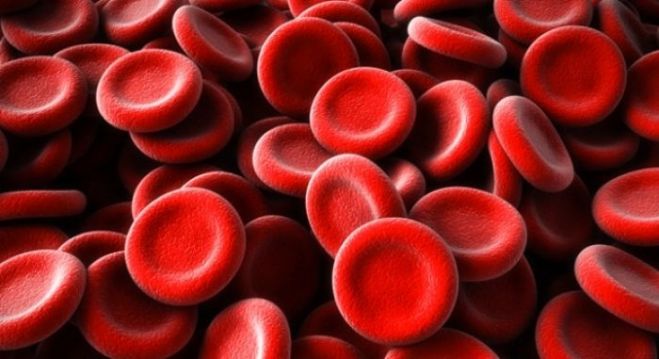
Red Blood Cells (RBC's)
Platelets Count
• Manual or microscopic method
• Automated method
Free-flowing capillary or well-mixed anticoagulated venous blood is added to a diluent at a specific volume in the Unopette reservoir. The diluents (1% ammonium oxalate) lyses the erythrocytes but preserves leukocytes and platelets. A 20 µL pipette is used with 1.98 ml of diluents to make a 1:100 dilution. The diluted blood is added to the hemacytometer chamber. Cells are allowed to settle for 10 minutes before leukocytes and platelets are counted. (Always refer to the manufacturer’s instructions for the procedure.)
Hemocytometer with cover glass, compound microscope. Unopette capillary pipette, lint-free wipe, alcohol pads, hand counter, petri dish with moist filter paper.
Ammonium oxalate: 11.45 gm
Sorensen’s phosphate buffer: 1.0 gm
Thimerosal: 0.1 gm
Distilled water: 1000 ml
EDTA-anticoagulated blood or capillary blood is preferred.
(1) Using the protective shield on the capillary pipette, puncture diaphragm of Unopette reservoir.
(2) Remove shield from pipette assembly by twisting. Holding pipette almost horizontally, touch tip of pipette to blood. Pipette will fill by capillary action. Filling will cease automatically when the blood reaches the end of the capillary bore in the neck of the pipette.
(4) Squeeze reservoir slightly to force out some air while simultaneously maintaining pressure on reservoir.
(5) Cover opening of overflow chamber of pipette with index finger and seat pipet securely in reservoir neck.
(6) Release pressure on reservoir. Then remove finger from pipette opening. At this time negative pressure will draw blood into reservoir.
(7) Squeeze reservoir gently two or three times to rinse capillary bore forcing diluent up int, but not out of, overflow chamber, releasing pressure each time to return mixture to reservoir.
(8) Place index finger over upper opening and gently invert several times to thoroughly mix blood with diuent.
(9) Cover overflow chamber with pipette shield and incubate at room temperature for 10 minutes before charging the hemacytometer.
(10) Meticulously clean the hemacytometer with alcohol or other cleaning solution. This is important because dust particles and other debris can be mistaken for platelets especially on a light microscope. Allow to dry completely before charging with diluted specimen.
(11) To charge the hemacyto-meter, convert to dropper assembly by withdrawing pipette from reservoir and reseating securely in reverse position.
(12) Invert reservoir and discard the first 3 or 4 drops of mixture.
(13) Carefully charge hemacyto-meter with diluted blood by gently squeezing sides of reservoir to expel contents until chamber is properly filled.
(14) Place hemacytometer in moist Petri dish for 10 minutes to allow platelets to settle. (Moistened filter paper retains evaporation of diluted specimen while standing.)
(15) Mount the hemacytometer on the microscope and lower its condenser.
(16) Procedure for counting platelets:
• Under 40x magnification, scan to ensure even distribution. Platelets are counted in all twenty-five small squares within the large center square. Platelets appear greenish, not refractile.
• Count cells starting in the upper left of the large middle square. Continue counting to the right hand square, drop down to the next row; continue counting in this fashion until the total area in that middle square (all 25 squares) have been counted.
• Count all cells that touch any of the upper and left lines, do not count any cell that touches a lower or right line.
• Count both sides of the hemocyt-ometer and take the average.
Ns x As x Ds
Total number of cells= 230
Dilution 1:100
Number of squares counted: 1
Area of each square: 1 mm3
Depth of solution: 0.1mm
cells/mm3 = 230 x 100
1 x 1 mm2 x 0.01 mm
= 230 x 103/L
• 150 - 450 x 109/L
REFERENCES
1. Brown, B.A., Haemotology, Principles and Procedures, Lea & Febiger, U.S.A., 1976.
2. Hoffbrand, A. V. and Pettit, 1. E., Essential Haemotology, Blackwell Scientific Publication, U.S.A., 1980.
3. Kassirsky, I. and Alexeev, G., Clinical Haemotology, Mir Publishers, U.S.S.R., 1972.
4. Widmann, F.K., Clinical interpretation of Laboratory tests, F.A. Davis Company, U.S.A., 1985.
5. Kirk, C.J.C. et al, Basic Medical Laboratory Technology, Pitman Book Ltd., U.K. 1982.
6. Green, J.H., An Introduction to human Physiology, Oxford University Press, U.K., 1980.
Total Leukocyte Count (TLC)
Total leukocyte count (TLC) refers to the number of white blood cells in 1 μl of blood (or in 1 liter of blood if the result is expressed in SI units). There are two methods for estimation of TLC:
- Manual or microscopic method
- Automated method
A differential leukocyte count should always be performed along with TLC to obtain the absolute cell counts.
The purpose of carrying out TLC is to detect increase or decrease in the total number of white cells in blood, i.e. leukocytosis or leukopenia respectively. TLC is carried out in the investigation of infections, any fever, hematologic disorders, malignancy, and for follow-up of chemotherapy or radiotherapy.
MANUAL METHOD
Principle
A sample of whole blood is mixed with a diluent, which lyses red cells and stains nuclei of white blood cells. White blood cells are counted in a hemocytometer counting chamber under the microscope and the result is expressed as total number of leukocytes per μl of blood or per liter of blood.
Equipment
(1) Hemocytometer or counting chamber with coverglass: The recommended hemocytometer is one with improved Neubauer rulings and metalized surface. There are two ruled areas on the surface of the chamber. Each ruled area is 3 mm × 3 mm in size and consists of 9 large squares with each large square measuring 1 mm × 1 mm. When the special thick coverglass is placed over the ruled area, the volume occupied by the diluted blood in each large square is 0.1 ml. In the improved Neubauer chamber, the central large square is divided into 25 squares, each of which is further subdivided into 16 small squares. A group of 16 small squares is separated by closely ruled triple lines. Metalized surface makes background rulings and cells easily visible. The 4 large corner squares are used for counting leukocytes, while the central large square is used for counting platelets and red blood cells. Only special coverglass, which is intended for use with hemocytometer, should be used. It should be thick and optically flat. When the special coverglass is placed on the surface of the chamber, a volumetric chamber with constant depth and volume throughout its entire area is formed. Ordinary coverslips should never be employed since they do not provide constant depth to the underlying chamber due to bowing.
When the special cover glass is placed over the ruled area of the chamber and pressed, Newton’s rings (colored refraction or rainbow colored rings) appear between the two glass surfaces; their formation indicates the correct placement of the cover glass.
(2) Pipette calibrated to deliver 20 μl (0.02 ml, 20 cmm): WBC bulb pipettes, which have a bulb for dilution and mixing (Thoma pipettes) are no longer recommended. This is because blood and diluting fluid cannot be mixed adequately inside the bulb of the pipette. Bulb pipettes are also difficult to calibrate, costly, and charging of counting chamber is difficult. Tips of pipettes often chip easily and unnecessarily small volume of blood needs to be used.
- Graduated pipette, 1 ml.
- Pasteur pipett
- Test tube (75 × 12 mm).
Reagent
WBC diluting fluid (Turk’s fluid) consists of a weak acid solution (which hemolyzes red cells) and gentian violet (which stains leucocyte nuclei deep violet). Diluting fluid also suspends and disperses the cells and facilitates counting. Its composition is as follows:
- Acetic acid, glacial 2 ml
- Gentian violet, 1% aqueous 1 ml
- Distilled water to make 100 ml
Specimen
EDTA anticoagulated venous blood or blood obtained by skin puncture is used. (Heparin should not be used since it causes leukocyte clumping). While collecting capillary blood from the finger, excess squeezing should be avoided so as not to dilute blood with tissue fluid.
Method
(1) Dilution of blood: Take 0.38 ml of diluting fluid in a test tube. To this, add exactly 20 μl of blood and mix. This produces 1:20 dilution. Alternatively, 0.1 ml of blood can be added to 1.9 ml of diluting fluid to get the same dilution.
(2) Charging the counting chamber: Place a coverglass over the hemocytometer. Draw some of the diluted blood in a Pasteur pipette. Holding the Pasteur pipette at an angle of 45° and placing its tip between the coverglass and the chamber, fill one of the ruled areas of the hemocytometer with the sample. The sample should cover the entire ruled area, should not contain air bubbles, and should not flow into the side channels. Allow 2 minutes for settling of cells.
(3) Counting the cells: Place the charged hemocytometer on the microscope stage. With the illumination reduced to give sufficient contrast, bring the rulings and the white cells under the focus of the low power objective (× 10). White cells appear as small black dots. Count the number of white cells in four large corner squares. (To reduce the error of distribution, counting of cells in all the nine squares is preferable). To correct for the random distribution of cells lying on the margins of the square, cells which are touching the left-hand lines or upper lines of the square are included in the count, while cells touching the lower and right margins are excluded.
(a) Calculation of TLC:
NLS
= Nw x 20 x 10
4
= Nw x 50
Where Nw is the number of WBCs counted, Cd is the correction of dilution, Cv is the correction of volume and NLS is the number of large squares counted.
(b) TLC/L = Number of WBCs counted × 50 × 106 (106 is the correction factor to convert count in 1 μl to count in 1 liter). Example: If 200 WBCs are counted in 4 large squares, TLC/μl will be 10,000/μl and TLC/liter will be 10.0 × 109/liter.
If TLC is more than 50,000/ml, then dilution of blood should be increased to 1:40 to increase the accuracy of the result.
If TLC is less than 2,000/ml then lesser dilution should be used.
Expression of TLC: Conventionally, TLC is expressed as cells/μl or cells/cmm or cells/mm3. In SI units, TLC is expressed as cells × 109/liter. Conversion factors for conventional to SI units is 0.001 and SI to conventional units is 1000.
Correction of TLC for nucleated red cells: The diluting fluid does not lyse nucleated red cells or erythroblasts. Therefore, they are counted as leukocytes in hemocytometer. If erythroblasts are markedly increased in the blood sample, overestimation of TLC can occur. To avoid this if erythroblasts are greater than 10 per 100 leukocytes as seen on blood film, TLC should be corrected for nucleated red cells by the following formula:
NRBC + 100
Where CTLC is the Corrected TLC/μl, TLC is the Total Leukocyte Count and NRBC is the Nucleated RBCs per 100 WBCs.
REFERENCE RANGES
- Adults 4000-11,000/μl
- At birth 10,000-26000/μl
- 1 year 6,000-16,000/μl
- 6-12 year 5,000-13,000/μl
- Pregnancy up to 15,000/μl
CRITICAL VALUES
- TLC < 2000/μl or > 50000/μl