Displaying items by tag: Hemotological Tests
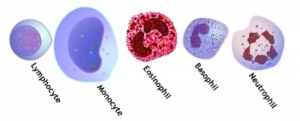
DIFFERENTIAL LEUKOCYTE COUNT (MANUAL METHOD)
METHOD
- Scan the slide in a methodical grid pattern, in order not to cover the same area twice. Counts can be completed quickly under 400×magnification, but if you are also evaluating morphology, 1000×magnification should be used.
- Count a minimum of 100 WBCs.
TOTAL ERYTHROCYTE COUNT (MANUAL METHOD)
Erythrocyte (Gr. erythros, red; kytos, cell) or red blood corpuscles are circular, anucleated, highly flexible, biconcave disc-shaped cells with high edges. The sixe of each cell averages 7.2 micrometer in diameter and 2.1 micrometer in thickness. It is 1.0 micrometer thick in the center. A complex membrane surrounds it, which is a bimolecular layer of protein. There is an inner most structure, called stroma, which is composed of lipids and proteins in the form of a fibrous protein. The cell contents are 90% hemoglobin. There are two methods for estimation of erythrocyte count:
- Manual or microscopic method
- Automated method
MANUAL METHOD
Equipment
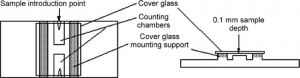
Hemocytometer with cover glass, compound microscope.
Reagent
Hayem’s diluting solution is prepared as follows:
- HgCl2 0.05 gm
- NaSO4 2.5 gm
- NaCl 0.5 gm
- Distilled water 100 ml
Specimen
EDTA anticoagulated venous blood or blood obtained by skin puncture is used.
Method
- Wipe finger with cotton soaked with alcohol, with a sterile lancet do small prick on the finger tip. Use pipette. Aspirate blood to 0.5.
- Aspirate diluting Hayem’s solution to the 101 mark. It will give 1:200 dilution of the blood.
- Hold the pipette horizontally and role it with both hands between finger and thumb.
- Place the counting chamber, absolutely free from dust and grease, on the table and lay the cover glass in place over the ruled area.
- Discard the first two or three drops from the pipette. Charge the counting chamber by holding the pipette in an inclined position. Allow 3 minutes for the cells to settle.
- Locate the central square, which is divided into 25 medium sized squares. Each of the medium sized squares is further divided into 16 smallest squares.
- Count the erythrocytes in medium sized squares (80 smallest squares) using high power objective.
- In order to avoid confusion in counting, count all cells wihich touch the upper and left outer double line of the group of 16 squares as if they were inside the square. Neglect all those cells, which touch the lower and right inner line.
Calculation
You may calculate total number of erythrocytes per cu mm of the blood as shown in the following.
Supose number of erythrocytes counted in 5 intermediate squares
= 1/5 sq mm
= 1/5 sqmm x 1/10 mm
= 1/50 cu mm
GENERAL NOTES
(1) Increased in numbers of RBC called polycythemia it is due to
• Bone marrow failure
• Erythropoietin deficiency (2ndry to kidney disease)
• Hemolysis (RBC destruction) from transfusion reaction
• Hemorrhage
• Leukemia
• Multiple myloma
• Nutritional deficiencies of (Iron, Copper, Folate, Vit B12, B6)
REFERENCE RANGES
- Newborns 4.8-7.2 millions
- Children 3.8-5.5 millions
- Adult (Male) 4.6-6.0 millions
- Adult (Female) 4.2-5.0 millions
- Pregnancy slightly lower than normal
MICRO METHOD FOR ESTIMATION OF PACKED CELL VOLUME (PCV) OR HEMATOCRIT
Principle
Anticoagulated whole blood is centrifuged in a capillary tube of uniform bore to pack the red cells. Centrifugation is done in a special microhematocrit centrifuge till packing of red cells is as complete as possible. The reading (length of packed red cells and total length of the column) is taken using a microhematocrit reader, a ruler, or arithmetic graph paper.
Equipment
- Microhematocrit centrifuge: It should provide relative centrifugal force of 12000 g for 5 minutes.
- Capillary hematocrit tubes: These are disposable glass tubes 75 mm in length and 1 mm in internal diameter. They are of two types: plain (containing no anticoagulant) and heparinised (coated with a dried film of 2 units of heparin). For plain tubes, anticoagulated venous blood is needed. Heparinised tubes are used for blood obtained from skin puncture.
- Tube sealant like plastic sealant or modeling clay; if not available, a spirit lamp for heat sealing.
- Microhematocrit reader; if not available, a ruler or arithmetic graph paper.
Specimen
Venous blood collected in EDTA (dipotassium salt) for plain tubes or blood from skin puncture collected directly in heparinised tubes. Venous blood should be collected with minimal stasis to avoid hemoconcentration and false rise in PCV.
Method
- Fill the capillary tube by applying its tip to the blood (either from skin puncture or anticoagulated venous blood, depending on the type of tube used). About 2/3rds to 3/4ths length of the capillary tube should be filled with blood.
- Seal the other end of the capillary tube (which was not in contact with blood) with a plastic sealant. If it is not available, heatseal the tube using a spirit lamp.
- The filled tubes are placed in the radial grooves of the centrifuge with the sealed ends toward the outer rim gasket. Counterbalance by placing the tubes in the grooves opposite to each other.
- Centrifuge at relative centrifu-gal force 12000 g for 5 minutes to completely pack the red cells.
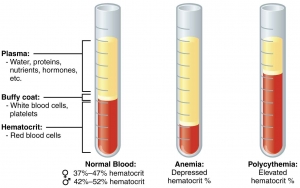
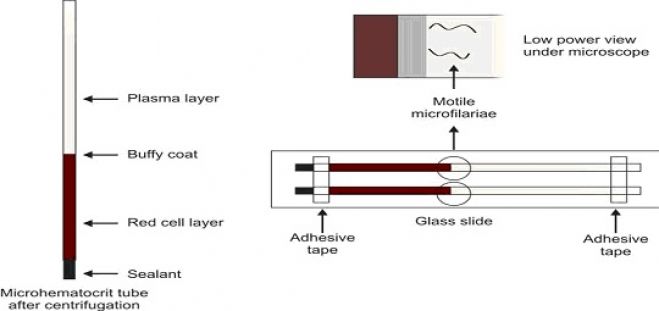
- Immediately remove the tubes from the centrifuge and stand them upright. The tube will show three layers from top to bottom: column of plasma, thin layer of buffy coat, and column of red cells.
- With the microhematocrit reader, hematocrit is directly read from the scale. If hematocrit reader is not available, the tube is held against a ruler and the hematocrit is obtained by the following formula:
Length of total column in mm
To obtain PCV, the above result is multiplied by 100.
GENERAL NOTES
- Prolonged application of tourniquet during venepuncture causes hemoconcentration and rise in hematocrit.
- Excess squeezing of the finger during skin puncture dilutes the sample with tissue fluid and lowers the hematocrit.
- Correct proportion of blood with anticoagulant should be used. Excess EDTA causes shrinkage of red cells and falsely lowers the hematocrit.
- Inadequate mixing of blood with anticoagulant, and inadequate mixing of blood before testing can cause false results.
- Low hematocrit can result if there are clots in the sample.
- Centrifugation at lower speed and for less time falsely increases PCV.
- A small amount of plasma is trapped in the lower part of the red cell column which is usually insignificant. Increased amount of plasma is trapped in microcytosis, macrocytosis, spherocytosis, and sickle cell anemia, which cause an artifactual rise in hematocrit. Larger volume of plasma is trapped in Wintrobe tube than in capillary tube.
- As PCV requires whole blood sample, it is affected by plasma volume (e.g. PCV is higher in dehydration, and lower in fluid overload).
- Expression of PCV: Occasionally, PCV is expressed as a percentage. In SI units, PCV is expressed as a volume fraction. Conversion factor from conventional to SI units is 0.1 and from SI to conventional units is 100.
- Rules of 3 and 9: These rules of thumb are commonly used to check the accuracy of results and are applicable only if red cells are of normal size and shape.
• Hemoglobin (gm/dl) × 3 = PCV
• Red cell count (million/cmm) × 9 = PCV - Automated hematocrit: In automated hematology analyzers, hematocrit is obtained by multiplying red cell count (in millions/cmm) by mean cell volume (in femtoliters).
REFERENCE RANGES
- Adult males: 40-50%
- Adult females (nonpregnant): 38 45%
- Adult females (pregnant): 36-42%
- Children 6 to 12 years: 37-46%
- Children 6 months to 6 years: 36 42%
- Infants 2 to 6 months: 32-42%
- Newborns: 44-60%
CRITICAL VALUES
- Packed cell volume: < 20% or > 60%
MACRO METHOD (WINTROBE METHOD) FOR ESTIMATION OF PACKED CELL VOLUME (PCV) OR HEMATOCRIT
Principle
Anticoagulated whole blood is centrifuged in a Wintrobe tube to completely pack the red cells. The volume of packed red cells is read directly from the tube. An advantage with this method is that before performing PCV, test for erythrocyte sedimentation rate can be set up.
Equipment
- Wintrobe tube: This tube is about 110 mm in length and has 100 markings, each at the interval of 1 mm. Internal diameter is 3 mm. It can hold about 3 ml of blood.
- Pasteur pipette with a rubber bulb and a sufficient length of capillary to reach the bottom of the Wintrobe tube.
- Centrifuge with a speed of 2300 g.
Specimen
Venous blood collected in EDTA (1.5 mg EDTA for 1 ml of blood) or in double oxalate. Test should be performed within 6 hours of collection.
Method
- Mix the anticoagulated blood sample thoroughly.
- Draw the blood sample in a Pasteur pipette and introduce the pipette up to the bottom of the Wintrobe tube. Fill the tube from the bottom exactly up to the 100 mark. During filling, tip of the pipette is raised, but should remain under the rising meniscus to avoid foaming.
- Centrifuge the sample at 2300 g for 30 min (To counterbalance a second Wintrobe tube filled with blood from another patient or water should be placed in the centrifuge).
- Take the reading of the length of the column of red cells.
Hematocrit can be expressed either as a percentage or as a fraction of the total volume of blood sample.
Significance
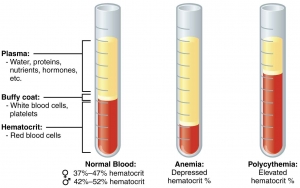
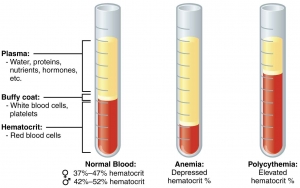
In anemia, PCV is below the lower level of normal range. PCV is raised in dehydration, shock, burns, and polycythemia.
After centrifugation of anticoagulated whole blood, three zones can be distinguished in the Wintrobe tube from above downwards-plasma, buffy coat layer (a small greyish layer of white cells and platelets, about 1 mm thick), and packed red cells. Normal plasma is straw-colored. It is colorless in iron deficiency anemia, pink in the presence of hemolysis or hemoglobinemia, and yellow if serum bilirubin is raised (jaundice). In hypertriglyceridemia, plasma appears milky. Increased thickness of buffy coat layer occur if white cells or platelets are increased in number (e.g. in leukocytosis, thrombocytosis, or leukemia). Smears can be made from the buffy coat layer for demonstration of lupus erythematosus (LE) cells, malaria parasites, or immature cells.
WHAT IS PACKED CELL VOLUME (PCV) OR HEMATOCRIT? USES AND METHODS FOR ESTIMATION OF PACKED CELL VOLUME (PCV)
Packed cell volume (PCV) is the volume occupied by the red cells when a sample of anticoagulated blood is centrifuged. It indicates relative proportion of red cells to plasma. PCV is also called as hematocrit or erythrocyte volume fraction. It is expressed either as a percentage of original volume of blood or as a decimal fraction.
USES OF PCV
- Detection of presence or absence of anemia or polycythemia
- Estimation of red cell indices (mean cell volume and mean corpuscular hemoglobin concentration)
- Checking accuracy of hemoglobin value (Hemoglobin in grams/dl × 3 = PCV).
There are two methods for estimation of PCV: macro method (Wintrobe method) and micro method (microhematocrit method). Micro method is preferred because it is rapid, convenient, requires only a small amount of blood, capillary blood from skin puncture can be used, and a large number of samples can be tested at one time.
This method is also more accurate as plasma trapping in red cell column is less.
SPECIFIC GRAVITY METHOD FOR ESTIMATION OF HEMOGLOBIN
By this method, the approximate value of hemoglobin is estimated. This method is simple and rapid. This method is most common in the blood bank for the selection of blood donors.
In this method, a drop of the blood sample is allowed to fall in the solution of copper sulfate having specific gravity 1.053 from the altitude of 1 cm. The hemoglobin concentration of 12.5 g/dl is equivalent to the specific gravity of 1.053. The drop of blood gets covered with copper proteinate and remains separate and distinct for 15-20 seconds. If the drop of blood sample sinks within 15-20 seconds, the specific gravity of copper sulfate solution is lower than the specific gravity of blood sample and the approximate value of hemoglobin is more than 12.5 grams/dl and hemoglobin level is acceptable for the donation of blood. If the drop of blood sample floats, hemoglobin value is less than 12.5 grams/dl and unacceptable for blood donation. However, the concentration of plasma proteins and total leukocyte count also influence the specific gravity of whole blood which may lead false-positive result. In the existence of hypergammaglobulinemia (e.g. multiple myeloma) or leukocytosis (e.g. myeloid or lymphoid reaction, chronic myeloid or lymphocytic leukemia), hemoglobin level will be misleadingly high.
OXYHEMOGLOBIN METHOD FOR ESTIMATION OF HEMOGLOBIN
For the estimation of hemoglobin by oxyhemoglobin method, blood sample is mixed with a weak ammonia solution and then absorbance of this solution is deliberated in a photometer using a yellow-green filter or measured in a spectrophotometer at 540 nanometer. Absorbance of the test sample is corresponded with that of the standard solution.For the estimation of hemoglobin by oxyhemoglobin method, blood sample is mixed with a weak ammonia solution and then absorbance of this solution is deliberated in a photometer using a yellow-green filter or measured in a spectrophotometer at 540 nanometer. Absorbance of the test sample is corresponded with that of the standard solution.
This method is much similar to cyanmethemoglobin (hemoglobin-cyanide) method.
This method is very simple and rapid but this method is not much reliable as compared to cyanmethemoglobin method because there is no stable standard solution is available, derivatives of hemoglobin except oxyhemoglobin are not measured, and color of oxyhemoglobin solution swiftly dims.
Microscopic Examination of Blood for Demonstration of Microfilaria
Since some species exhibit periodicity (i.e. circulation of microfilariae in increased numbers at certain times of the day), blood should be collected at the correct time to improve the chances of detection. For Wuchereria bancrofti and Brugia malayi showing nocturnal periodicity, blood should be collected at night between 10 p.m. to 4 a.m. Microfilariae are present in greater numbers in capillary blood than in venous blood; therefore, skin puncture is preferred. Usually microfilariae are scanty in peripheral blood so that concentration techniques may be necessary for their demonstration.
Cyanmethemoglobin (Hemoglobin-Cyanide) Method for Estimation of Hemoglobin
When Blood is mixed with a solution of potassium cyanide, potassium ferricyanide, and Drabkin’s solution, the erythrocytes are lysed by producing evenly disturbed hemoglobin solution. Potassium ferricyanide transforms hemoglobin into methemoglobin, and methemoglobin combines with potassium cyanide to produce hemiglobincyanide (cyanmethemoglobin). This method is optional for the estimation of hemoglobin and this method is recommended by the International Committee for Standardization in hemotology. This is because in this method all type of hemoglobin is transformed to cyanmethemoglobin (except sulfhemoglobin), and a firm and trustworthy standard is available.
- Cell Biology
- Microbiology
- Hemotology
- Zoology
- Article
- How to
- Hemotological Tests
- Cyanmethemoglobin Method
- Cyanmethemoglobin Method Procedure
- Cyanmethemoglobin Apparatus
- Cyanmethemoglobin Reagent
- Cyanmethemoglobin Standard
- Cyanmethemoglobin Standard Solution
- Cyanmethemoglobin Method Sources of Error
- Cyanmethemoglobin MSDS
- Cyanmethemoglobin Method Hemoglobin Estimation
Sahli’s Acid Hematin Method for the Estimation of Hemoglobin
When blood is mixed with an acid solution, the hemoglobin converts into the brown-colored acid hematin. The acid hematin is then diluted with distilled water till the color of the acid hematin matches that of the brown glass standard. The hemoglobin is estimated by reading the value directly from the scale.
What is Hemoglobin? Types, Indications and Methods
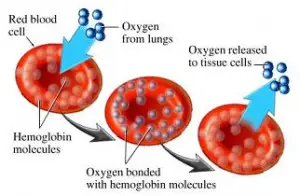
Hemoglobin is composed of heme (iron + protoporphyrin) and globin polypeptide chains. It is present in the red blood cells of all vertebrates except Channichthyidae (the family of fish: white-blooded fish also called crocodile fish found in southern South America and the Southern Ocean around Antarctica). It carries oxygen from the lungs to the tissues and carbon dioxide from tissues to the lungs.
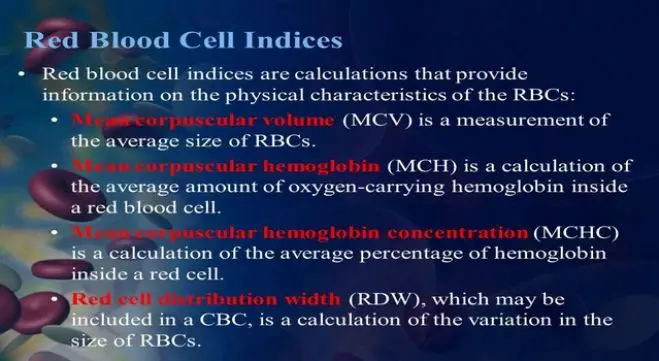
Red Cell Indices
USES OF RED CELL INDICES
(2) Differentiation of iron deficiency anemia from thalassemia trait: In iron deficiency, MCV, MCH, and MCHC are low, while in thalassemia trait, MCV and MCH are low and MCHC is normal.
RBC count in million/cmm
• Non-megaloblastic macrocytosis: Chronic alcoholism, liver disease, hypothyroidism, normal pregnancy, reticulocytosis
• Newborns.
In the presence of large number of abnormal red cells like sickle cells, and in dimorphic anemia (e.g. mixed normocytic and microcytic), MCV may be normal (since it is an average value) and thus unreliable for morphological classification.
Mentzer index is derived by dividing MCV with red cell count. Ratio of less than 13 is seen in thalassemia while ratio is more than 13 in iron deficiency anemia.
RBC count in millions/cmm
MCH is decreased in microcytic hypochromic anemia, and increased in macrocytic anemia and in newborns.
PCV in %
• Mean cell hemoglobin: 27-32 pg
• Mean cell hemoglobin concentra-tion: 30-35 g/dl
• Red cell distribution width: 9.0-14.5
1. Henry JB. Clinical diagnosis and management by laboratory methods (20th Ed). Philadelphia: WB Saunders Company, 2001.
2. Wallach J. Interpretation of Diagnostic Tests (7th Ed). Philadelphia: Lippincott Williams and Wilkins, 2000⁻¹⁵
Cross Match Procedure in Blood Bank (Manual Method)
When the recipient’s ABO and Rh blood groups are determined, the donor blood unit that is ABO and Rh compatible is selected, and compatibility test is carried out. The purpose of compatibility test is to prevent the transfusion of incompatible red cell units and thus avoidance of hemolytic transfusion reaction in the recipient. Compatibility test detects (i) major ABO grouping error, and (ii) most clinically significant antibodies reactive against donor red cells.
There are two types of cross-match: major cross-match (testing recipient’s serum against donor’s red cells) and minor cross-match (testing donor’s serum against recipient’s red cells). However, minor cross-match is considered as less important since antibodies in donor blood unit get diluted or neutralized in recipient’s plasma. Also, if antibody screening and identification is being carried out, minor cross-matching is not essential. Therefore, only the red cells from the donor unit are tested against the recipient’s serum and the name compatibility test has replaced the term cross-matching. For transfusion of platelets or fresh frozen plasma, cross-matching is not required. However, fresh frozen plasma should be ABO-compatible.
A full cross-matching procedure consists of:
- Immediate spin cross-match at room temperature, and
- Indirect antiglobulin test at 37°C.
IMMEDIATE SPIN CROSS MATCH
The purpose of this test is to detect ABO incompatibility. Equal volumes of 2% saline suspension of red cells of donor and recipient’s serum are mixed, incubated at room temperature for 5 minutes, and centrifuged. Agglutination or hemolysis indicates incompatibility.
Causes of False-negative Test
- A2B donor red cells and group B recipient serum.
- Rapid complement fixation of potent ABO antibodies with bound complement interfering with agglutination.
Causes of False-positive Test
- Rouleaux formation
- Cold-reactive antibodies: If agglutination disappears by keeping the tube at 37°C for 10 minutes, presence of cold agglutinins is confirmed.
INDIRECT ANTIGLOBULIN TEST
Saline-suspended red cells of the donor after being incubated in patient’s serum are washed in saline and antiglobulin reagent is added. Following re-centrifugation, examine for agglutination or hemolysis. This test detects most of the clinically significant IgG antibodies.
If agglutination or hemolysis is not observed in any of the above stages, donor unit is compatible with recipient’s serum. Agglutination or hemolysis at any stage is indicative of incompatibility.
EMERGENCY CROSS-MATCH
If blood is required urgently, ABO and Rh grouping are carried out by rapid slide test and immediate spin cross match (i.e. the first stage of cross match) is performed (to exclude ABO incompatibility). If the blood unit is compatible, then after issuing it, remaining stage of the cross-match is completed. If any incompatibility is detected, the concerned physician is immediately informed about the incompatibility detected.
ANTIBODY SCREENING AND IDENTIFICATION
Screening for unexpected or irregular antibodies is done during pre-transfusion testing in recipient’s serum and in donor’s blood. In this test, serum of the recipient is tested against a set of three group O screening cells of known antigenic type. If unexpected antibodies are detected, then they are identified and blood unit that lacks the corresponding antigen is selected for compatibility test.
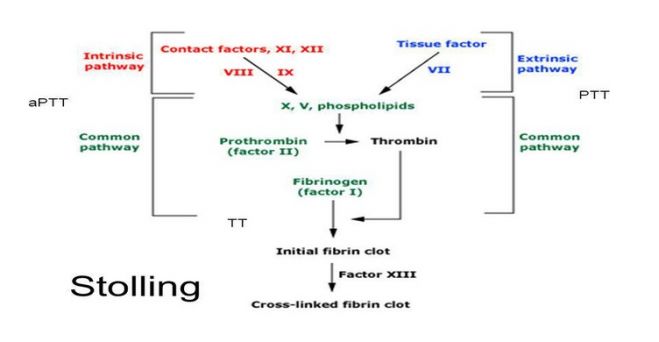
Activated Partial Thromboplastin Time (APTT)
Plasma is incubated with an activator (which initiates intrinsic pathway of coagulation by contact activation). Phospholipid (also called as partial thromboplastin) and calcium are then added and clotting time is measured.
(1) Kaolin 5 gm/liter: This is a contact activator.
(2) Phospholipid: Various APTT reagents are available commercially, which contain phospholipids.
(3) Calcium chloride 0.025 mol/liter.
(1) Mix equal volumes of phospholipid reagent and calcium chloride solution in a glass test tube and keep in a waterbath at 37°C.
(2) Deliver 0.1 ml of plasma in another test tube and add 0.1 ml of kaolin solution. Incubate at 37°C in the waterbath for 10 minutes.
(3) After exactly 10 minutes, add 0.2 ml of phospholipidcalcium chloride mixture, start the stopwatch, and note the clotting time.
30-40 seconds.
(1) Hemophilia A or B.
(2) Deficiencies of other coagulation factors in intrinsic and common pathways.
(3) Presence of coagulation inhibitors
(4) Heparin therapy
(5) Disseminated intravascular coagulation
(6) Liver disease
(1) Screening for hereditary disorders of coagulation: Since deficiencies of F VIII (hemophilia A) and F IX (hemophilia B) are relatively common, APTT is the most important screening test for inherited coagulation disorders. APTT detects deficiencies of all coagulation factors except F VII and F XIII. PT is also performed along with APTT. Prolongation of both PT and APTT is indicative of deficiency of coagulation factors in common pathway. Normal PT with prolongation of APTT is indicative of intrinsic pathway deficiency (particularly of F VIII or IX).
(2) To monitor heparin therapy: Heparin potentiates the action of natural anticoagulant antithrombin III which is an inhibitor of thrombin and activated factors IX, X, and XI. Full dose heparin therapy needs monitoring by APTT to maintain the dose in the therapeutic range (1.5 to 2.5 times the upper reference limit of APTT).
(3) Screening for circulating inhibitors of coagulation: APTT is prolonged in the presence of specific inhibitors (which are directed against specific coagulation factors) and non-specific inhibitors (which interfere with certain coagulation reactions).
Prothrombin Time (PT)
Tissue thromboplastin and calcium are added to plasma and clotting time is determined. The test determines the overall efficiency of extrinsic and common pathways.
(1) Water bath at 37°C
(2) Test tubes (75 × 12 mm)
(3) Stopwatch
(1) Thromboplastin reagent: This contains tissue factor and phospholipids and is available commercially.
(2) Calcium chloride 0.025 mol/liter.
Venous blood is collected from antecubital fossa with a plastic, siliconized glass, or polypropylene syringe and a large bore needle (20 or 21 G in adults, 22 or 23 G in infants). Blood should never be collected from indwelling intravenous lines, as these often contain heparin. Glass syringe should not be used for collection since it activates coagulation. The blood is drawn gently but quickly after a single, smooth venepuncture. The needle is detached from the syringe, and the sample is passed gently into the plastic container. After capping the container, the blood and the anticoagulant are mixed immediately by gentle inversion 5 times. The anticoagulant used for coagulation studies is trisodium citrate (3.2%), with anticoagulant to blood proportion being 1:9. Most coagulation studies require platelet poor plasma (PPP). To obtain PPP, blood sample is centrifuged at 3000-4000 revolutions per minute for 15-30 minutes. Coagulation studies are carried out within 2 hours of collection of sample.
(1) Deliver 0.1 ml of plasma in a glass test tube kept in water bath at 37°C.
(2) Add 0.1 ml of thromboplastin reagent and mix.
(3) After 1 minute, add 0.1 ml of calcium chloride solution. Immediately start the stopwatch and record the time required for clot formation.
11-16 seconds.
(1) Treatment with oral anticoagulants
(2) Liver disease
(3) Vitamin K deficiency
(4) Disseminated intravascular coagulation
(1) To monitor patients who are on oral anticoagulant therapy: PT is the standard test for monitoring treatment with oral anticoagulants. Oral anticoagulants inhibit carboxylation of vitamin K-dependent factors (Factors II, VII, IX, and X) and make these factors inactive.
In patients receiving oral anticoagulants, PT should be reported as a ratio of PT of patient to PT of control; it should not be reported as percentage. Various types of thromboplastin reagents obtained from different sources (like ox brain, rabbit brain, or rabbit lung) are available for PT test. These differ in their responsiveness to deficiency of vit. K-dependent factors. Technique of PT is also different in different laboratories. For standardization and to obtain comparable results, it is recommended to report PT (in persons on oral anticoagulants) in the form of an International Normalized Ratio (INR).
INR = PT of Patient ISI
PT of Control
(2) To assess liver function: Liver is the site of synthesis of various coagulation factors, including vitamin Kdependent proteins. Therefore PT is a sensitive test for assessment of liver function.
(3) Detection of vitamin K deficiency: PT measures three of the four vitamin K-dependent factors (i.e. II, VII, and X).
(4) To screen for hereditary deficiency of coagulation factors VII, X, V, prothrombin, and fibrinogen.
Bleeding Time (BT) and Clotting Time (CT)
The Bleeding and Clotting time test refers to a test that is performed on a sample of blood to measure the time taken for it to clot or coagulate. This test is also known as the BT CT test.
BLEEDING TIME
The bleeding time test is dependent on appropriate functioning of platelets blood vessels and platelets and evaluates earliest hemostasis (platelets components and vascular).
In this test, incision (a surgical cut made in skin) or a superficial skin puncture is made and the time is measured for bleeding to stop.
There are three methods most commonly used to measure bleeding time:
- Duke’s method
- Ivy’s method
- Template method
In Duke’s method, ear lobe is puncture, and the time is measured for bleeding to stop. This method is not recommended and cannot be standardized because it can cause a large local hematoma. In Ivy’s method, on the volar surface of the forearm, three punctures are made with a lancet (cutting depth 2-2.5 mm) under normal pulse pressure (between 30-40 mm Hg). A disadvantage of Ivy’s method is closure of puncture wound before stoppage of bleeding. In Template method, a special surgical blade is uses to make a larger cut of about 1 mm deep and 5 mm long. Although Template method is better than other methods, it may produce large scar and even form a keloid (irregular fibrous tissue formed at the site of a scar) in predisposed individuals. Ivy’s method for the measurement of bleeding time is described below.
Ivy’s Method
Principle:
On the volar surface of forearm, three normal punctures are made with the help of a lancet under normal pulse pressure (between 30-40 mm Hg). The average time is measured for bleeding to stop from the puncture sites.
Equipment
- Disposable sterile lancets
- Sphygmomanometer
- Filter paper
- Stopwatch
Method
- Blood pressure of the patient is measured with the help of sphygmomanometer. The blood pressure of the patient should be normal before going to the further process.
- The volar surface of the forearm is cleansed with ethanol 70% and allowed to dry.
- With the help of a lancet, in quick succession, three punctures are made about 5 cm apart. Note that scars and superficial veins should be avoided.
- Start the stopwatch as soon as puncture made on the volar surface of the forearm.
- With the help of the filter paper, blood oozing from the puncture wound is gently absorbed with intervals of 15 seconds.
- The timer is stopped when blood no more mark the filter paper.
- Time measured for bleeding to stop from all the three puncture wound is recorded. The average time is calculated and reported as the bleeding time.
Reference Ranges
- Normal range: 2-7 minutes.
- The greater numbers of individuals have bleeding time less than 4 minutes. The bleeding time should be reported in minutes or nearest half minute. If the bleeding continues more than twenty minutes, the test is stopped and the bleeding time should be reported as >20 minutes (more than 20 minutes).
Cause of extend of duration of bleeding time
- Disorders of blood vessels
- Thrombocytopenia: This term is uses when the platelet count is less than its normal value. The bleeding time test should not be performed if the platelet count is less than 1,00,000/ml. It may be difficult to control the bleeding if the platelet count is very low.
- Von Willebrand disease
- Disorder of platelet function
- Afibrinogenemia
CLOTTING TIME
In this test, required time is measured for the blood to clot in a glass test tube, kept at 37° C. Extend of duration of clotting time occurs only if severe deficiency of a clotting factor exists and is normal in moderate or mild deficiency.