Periodic Acid-Schiff (PAS) Stain: Purpose, Principle, Procedure, and Interpretation
Periodic Acid-Schiff (PAS) stain detects carbohydrates, fungi, glycogen. Learn purpose, procedure, interpretation for diagnosing fungal infections, glycogen storage disorders, and tumors.
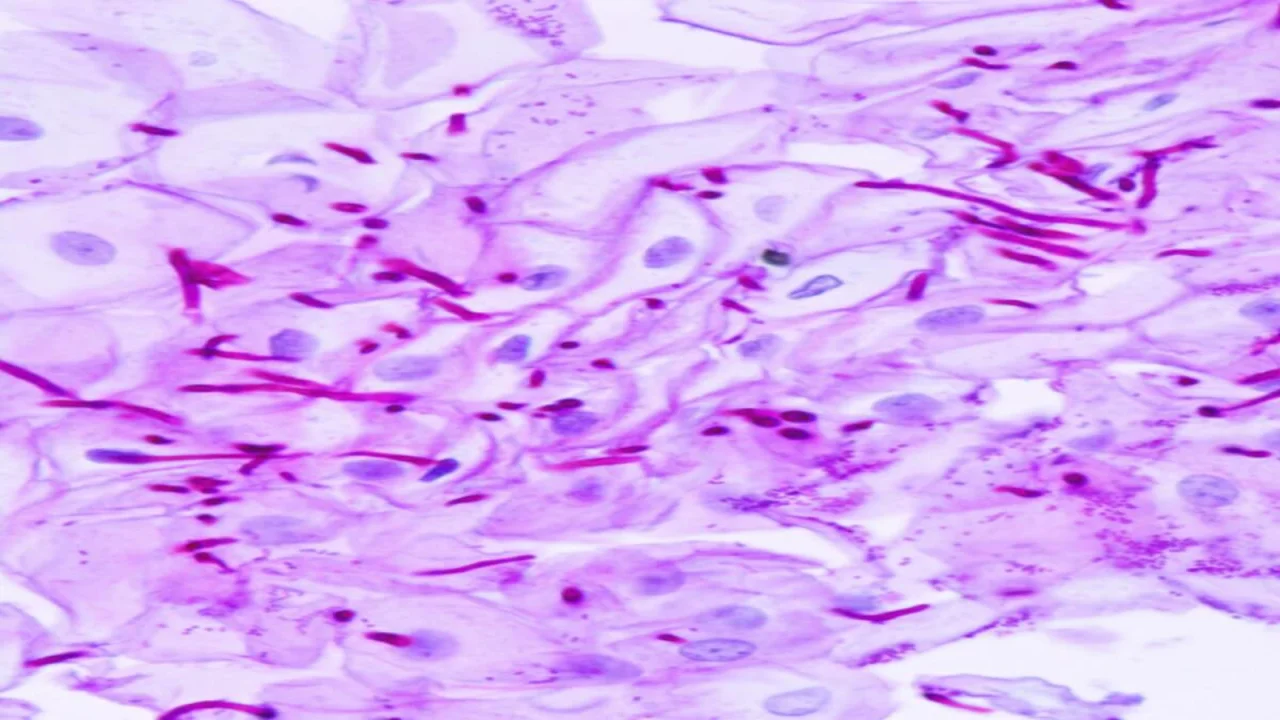
The Periodic Acid-Schiff (PAS) stain is a histochemical method used in histopathology to detect carbohydrates and carbohydrate-containing macromolecules in tissues, including polysaccharides (e.g., glycogen), mucosubstances (glycoproteins, glycolipids, mucins), basement membranes, and fungi. It is critical for diagnosing fungal infections, glycogen storage disorders, and epithelial tumors by highlighting abnormal carbohydrate deposits.
Diagnostic Applications of Periodic Acid-Schiff (PAS) Stain
1. Glycogen and Polysaccharide Detection
Role: Identifies glycogen deposits in tissues, critical for diagnosing glycogen storage diseases (e.g., von Gierke disease) and assessing hepatic abnormalities.
Clinical Relevance: Differentiates glycogen accumulation from other intracellular inclusions in liver biopsies.
2. Basement Membrane Evaluation
Role: Visualizes basement membrane thickness, particularly in renal glomeruli, aiding in the diagnosis of renal diseases like diabetic nephropathy or membranous glomerulonephritis.
Clinical Relevance: Highlights structural abnormalities in kidney biopsies for targeted treatment planning.
3. Fungal Infection Identification
Role: Detects fungi (e.g., Cryptococcus neoformans, Histoplasma capsulatum, Aspergillus fumigatus) by staining polysaccharides in their cell walls or capsules.
Clinical Relevance: Supports rapid diagnosis of invasive fungal infections in immunocompromised patients.
4. Mucin and Glandular Secretions
Role: Highlights acid mucins in glandular tissues (e.g., endocervical, intestinal, and bronchial glands).
Clinical Relevance: Distinguishes mucin-producing adenocarcinomas from other neoplasms.
5. Lysosomal Storage Disorders
Role: Demonstrates abnormal accumulations of cerebrosides and gangliosides in disorders like Gaucher’s disease and Krabbe’s disease.
Clinical Relevance: Confirms lipid metabolism defects in bone marrow or nerve tissue biopsies.
6. Pigment and Cellular Inclusion Analysis
Role: Stains lipofuscin (age-related pigment) and Dubin-Johnson syndrome pigments.
Clinical Relevance: Differentiates pathological pigments from benign deposits in liver or skin biopsies.
7. Plasma Cell Evaluation
Role: Identifies Russell bodies (abnormal immunoglobulin aggregates) in plasma cells.
Clinical Relevance: Aids in diagnosing chronic inflammatory conditions or plasma cell dyscrasias.
Principle of PAS Stain
The Principle of PAS Stain relies on a two-step chemical reaction:
- Oxidation: Periodic acid breaks 1,2-glycol groups in carbohydrates (e.g., glycogen, mucins) into reactive dialdehydes.
- Color Formation: Dialdehydes bind to Schiff’s reagent (fuchsin-sulfurous acid), forming a stable magenta-colored complex. This highlights carbohydrate-rich structures (e.g., basement membranes, fungi) in tissues, aiding histopathological diagnosis.
Preparation of Reagents for Periodic Acid-Schiff (PAS) Staining
- Periodic Acid Solution: Dissolve 1 gram of periodic acid in 100 milliliters of distilled water.
- Schiff’s Reagent: Dissolve basic fuchsin in boiling water, cool to 50°C, filter, add sodium metabisulphite and HCl, store overnight in darkness, then purify with activated charcoal and filter.
1. Periodic Acid Solution
The Periodic Acid Solution facilitates the oxidation phase of the PAS staining process, converting carbohydrates into aldehyde groups. While concentrations ranging from 0.5% to 2.5% are viable, a 1% solution is widely recommended for consistent results.
Ingredients:
- Periodic acid: 1 gram
- Distilled water: 100 milliliters
Preparation Steps:
- Measure 1 gram of periodic acid.
- Add it to 100 milliliters of distilled water.
- Stir the mixture thoroughly until the periodic acid fully dissolves.
This solution is now ready for use in the PAS staining protocol.
2. Schiff’s Reagent
Schiff’s Reagent is essential for detecting aldehydes formed during oxidation, producing a distinct magenta color that highlights carbohydrate-rich structures. Its preparation requires careful attention to detail.
Ingredients:
- Basic fuchsin: 1 gram
- Distilled water: 100 milliliters
- Sodium metabisulphite: 2 grams
- Concentrated hydrochloric acid (HCl): 2 milliliters
- Activated charcoal: 0.3 grams
Preparation Steps:
- Dissolve 1 gram of basic fuchsin in 100 milliliters of boiling distilled water, stirring until fully dissolved.
- Cool the solution to 50°C, then filter it to remove any residual particles.
- Add 2 grams of sodium metabisulphite and 2 milliliters of concentrated hydrochloric acid to the filtered solution. Stir well to combine.
- Store the mixture in a dark room at room temperature overnight to allow stabilization.
- The next day, add 0.3 grams of activated charcoal and shake the solution for one minute to adsorb impurities.
- Filter the solution again to yield the final Schiff’s Reagent.
This reagent is now prepared for use in the PAS staining process.
Procedure of Periodic Acid-Schiff (PAS) Stain
- Deparaffinization: Heat the slide over a flame and immerse it in xylene to dissolve the paraffin wax embedding the tissue. Repeat this step to ensure all wax is removed, allowing subsequent reagents to penetrate the sample effectively.
- Hydration: After removing the xylene, hydrate the tissue by passing it through a series of alcohol baths with decreasing concentrations (100%, 90%, 80%, 70%), followed by distilled water. This gradual process reintroduces water into the tissue for optimal staining.
- Oxidation: Submerge the tissue sections in a 1% periodic acid solution for 5 to 10 minutes. This step oxidizes carbohydrates into aldehydes, preparing them for the staining reaction.
- Rinse: Wash the sections with at least two changes of distilled water to eliminate residual periodic acid, preventing interference with the staining process.
- Treatment with Schiff’s Reagent: Apply Schiff’s reagent to the sections and let it sit for 20 to 30 minutes. The reagent reacts with the aldehydes, staining PAS-positive structures a distinct magenta color.
- Rinse: Rinse the sections under running tap water for 5 to 10 minutes to remove excess Schiff’s reagent, ensuring a clean background for observation.
- Counterstaining: Stain the sections with hematoxylin for 3 to 5 minutes, then differentiate and blue them. This counterstain enhances contrast by coloring the nuclei blue against the magenta PAS-positive areas.
- Dehydration: Dehydrate the tissue by passing it through alcohol solutions of increasing concentrations (70%, 80%, 90%, 100%). This removes water, preparing the sample for clearing.
- Clearing: Immerse the slides in two consecutive xylene baths to clear the tissue. This step removes alcohol and renders the tissue transparent for microscopic analysis.
- Mounting: Apply a mounting medium, such as DPX, to the sections and cover with a coverslip. This preserves the stained sample and protects it for examination.
- Observation: Examine the prepared slide under a light microscope. PAS-positive structures, such as carbohydrates, will appear magenta, contrasting with the blue counterstained background.
Note: Ensure proper ventilation when handling xylene.
Result and Interpretation
What Do PAS Stain Results Show?
When examining PAS-stained tissues under a microscope:
- PAS-positive materials appear magenta pink to red.
- Nuclei are stained blue.
These color changes highlight carbohydrate-containing structures, making it easier to distinguish them from other tissue elements.
PAS-Positive Substances: Categories and Examples
Numerous substances react positively with the PAS stain. Below is a detailed breakdown:
- Polysaccharides: Complex carbohydrates that include:
- Glycogen, found in many white blood cells (leucocytes).
- Cellulose and starch.
- Fungal capsules, such as those of Candida albicans, Histoplasma capsulatum, Cryptococcus, and Blastomyces.
- Actinomycosis and some bacteria with polysaccharide-rich walls.
- Glycoproteins: Proteins with carbohydrate attachments, such as:
- Mucins, present in secretions of the intestinal tract, uterine glands, and tracheobronchial trees.
- Hormones like thyroid-stimulating hormone (TSH).
- Components of megakaryocytes (cells involved in blood clotting).
- Glycolipids: Carbohydrate-linked lipids, including:
- Gangliosides, fatty acid-rich molecules prevalent in the brain’s gray matter.
- Non-Carbohydrate Containing Substances: Certain compounds without carbohydrates still react due to other chemical groups:
- Unsaturated lipids.
- Phospholipids.
- Phosphoinositides.
- Certain Pigments and Substances: Specific pigments that show PAS positivity:
- Ceroid.
- Lipofuscin.
- Pigment linked to melanosis coli.
- Dubin-Johnson pigment.
- Plasmogens: Acetyl phospholipids, such as:
- Russell bodies, found in plasma cells.
- Miscellaneous Substances: Additional PAS-positive materials include:
- Amyloid (abnormal protein deposits).
- Cartilage matrix.
- Colloid material.
- Ocular lens components.
Why Are These Substances PAS-Positive?
The PAS stain targets carbohydrates by oxidizing them into aldehydes, which then bind with Schiff’s reagent to produce the characteristic magenta color. Substances like polysaccharides, glycoproteins, and glycolipids contain carbohydrates that trigger this reaction. Other materials, such as certain lipids and pigments, may also react due to similar chemical properties, enhancing the stain’s diagnostic versatility.
Clinical Applications of PAS Interpretation
- Identifies glycogen storage disorders (e.g., Pompe disease)
- Detects fungal infections in tissue biopsies
- Highlights mucin-producing tumors (e.g., adenocarcinomas)
- Assesses renal basement membrane abnormalities
Note: Magenta staining confirms carbohydrate-rich structures. Always correlate findings with clinical history and ancillary tests for accurate diagnosis.
FAQs
What does the Periodic Acid-Schiff (PAS) stain do, and when is it positive?
What does the Periodic Acid-Schiff stain demonstrate?
What is the Periodic Acid-Schiff (PAS) stain used for, particularly in detecting fungi and other substances?
The information on this page is peer reviewed by a qualified editorial review board member. Learn more about us and our editorial process.
Last reviewed on .
Article history
- Latest version
Reference(s)
- Shariff, Shameem., et al. “Principles & Interpretation of Laboratory Practices in Surgical Pathology.”, JP Medical Ltd, 2016, isbn: 9789352500246.
- Dey, Pranab. “Basic and Advanced Laboratory Techniques in Histopathology and Cytology.”, Springer, 2018, isbn: 9789811082528.
- Bancroft, John D.., et al. “Theory and Practice of Histological Techniques.”, Elsevier Health Sciences, 2008, isbn: 9780443102790.
- Löffler, Helmut., et al. “Atlas of Clinical Hematology.”, Springer Science & Business Media, 2004, isbn: 9783540210139.
- Vyas, Monika., et al. “PAS (Periodic acid-Schiff).”, 15 July 2022 PathologyOutlines.com, Inc. <https://www.pathologyoutlines.com/topic/stainspas.html>.
- LabCE. “Periodic Acid-Schiff (PAS): Diagnostic Applications.” LabCE <https://www.labce.com/spg949466_periodic_acid_schiff_pas_diagnostic_applications.aspx>.
Cite this page:
- Posted by Dayyal Dungrela