Colorimeter: Principle, Uses, and How It Works
Colorimeter is a type of photometer used to measure solution concentration. Learn how it works, its components, and applications.
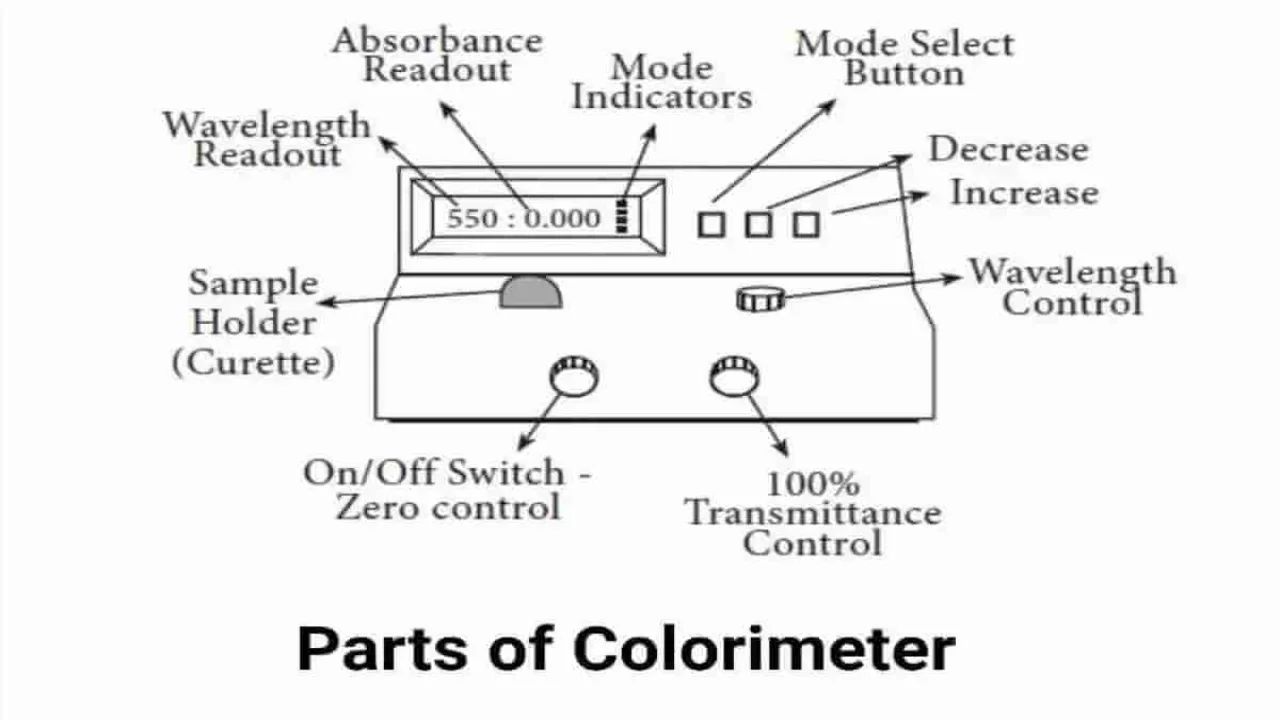
Colorimeter is a type of photometer used to measure the concentration of light-absorbing substances by measuring the light-transmitting ability of colored solutions. It was invented by Jules Duboscq in 1870 and named as “Duboscq calorimeter”. The intensity of the colored solute is estimated by comparing it with a standard solution of known concentration.
Principle of Colorimeter
When a beam of incident light with intensity I₀ passes through a solution, the following events occur:
- A portion of the incident light is reflected through the solution. It i s denoted as Iᵣ.
- A portion of the incident light is absorbed through the solution. It is denoted as Iₐ.
- The remaining light is transmitted through the solution. It is denoted as Iₜ.
Since Iᵣ is maintained constant by using cuvettes with identical optical properties, the focus is on the transmitted light. The light that is not absorbed passes through the solution and is responsible for the observed color. It’s important to note that the color of the incident light should be complementary to the color of the solution.
The ratio of transmitted light intensity (Iₜ) to the incident light intensity (I₀) is called transmittance (T). Photometric instruments are designed to measure this transmittance. Mathematically, it is expressed as:
The absorbance (A) of a solution at a specific wavelength is defined as the base-10 logarithm of the reciprocal of transmittance. That is:
These measurements rely on two important laws:
Beer’s Law
According to Beer’s law, when monochromatic light passes through a colored solution, the amount of light absorbed is directly proportional to the concentration (C) of the solute.
Lambert’s Law
According to Lambert’s law, when monochromatic light passes through a colored solution, the amount of light absorbed is directly proportional to the path length (L) or thickness of the solution.
Bear-Lambert’s Law
By combining the both Bear-Lambert’s law:
Absorbance (A) is proportional to the product of concentration and path length.
Where K is a constant and is known as the absorption coefficient.
Since the path length remains the same (using the same cuvette), the concentration of an unknown solution can be calculated by using the below equation:
Components of Colorimeter
- Light Source: The light source should emit energy with sufficient intensity across the entire visible spectrum (380–780 nm). A tungsten lamp is commonly used for this purpose.
- Slit: The slit allows a narrow beam of light to path and minimize unwanted light.
- Condensing Lens: It focuses the light into a parallel beam for optimal passage through the optical components.
- Monochromator: This component isolates a single wavelength band (monochromatic light) from the polychromatic (white) light emitted by the source. It allows only the desired wavelength to pass through. Prisms, gelatin filters, grating monochromators, or interference filters may be used for this purpose.
- Cuvette: It is used to hold the sample. The cuvette must be transparent. Glass or clear plastic cuvettes are preferred.
- Photo Detectors: The photo detector captures the light that passes through the sample and converts it into an electrical signal. Silicon photocells, selenium photocells, photomultiplier tubes and phototubes are commonly used.
- Display: It processes the electrical signal and presents the result as a visible output.
How is a Colorimeter Used?
- Calibrate the colorimeter using a blank solution (usually distilled water) to establish a baseline.
- Prepare your test sample and ensure it’s free of air bubbles and contamination.
- Place the sample in a cuvette and insert it into the device.
- Select the appropriate wavelength or filter that matches the target substance’s absorption peak.
- Record the displayed absorbance or transmittance value and compare it with standards.
Uses and Applications of Colorimeters
Colorimeters are used across a wide range of industries and scientific disciplines:
- Clinical Laboratories: In clinical laboratories, the colorimeter is commonly used to estimate various biochemical compounds in a range of biological samples such as blood, plasma, serum, cerebrospinal fluid (CSF), urine, and other body fluids. In all methods where a colored product is formed in reaction with a specific analyte, the analyte can be quantitatively measured. Colorimeters are also extensively used to monitor the growth of bacterial or yeast cells in liquid culture media.
- Food and Beverage Industry: In the food and beverage industry, colorimeters are used to assess color consistency and quality of products, monitor concentration of additives or nutrients, and ensure compliance with product standards.
- Environmental Science: In environmental science, colorimeters are applied to analyze water and soil samples for pollutants, such as nitrates, phosphates, and heavy metals, by measuring the intensity of color formed during chemical reactions, aiding in pollution control and environmental monitoring.
- Chemical Analysis: In chemical analysis, colorimeters are used to determine the concentration of substances in various chemical solutions by measuring the absorbance of light.
Colorimeter vs. Spectrophotometer
| Feature | Colorimeter | Spectrophotometer |
|---|---|---|
| Wavelength Range | Visible light only | UV, visible, and near-infrared |
| Complexity | Simple operation | More advanced and programmable |
| Cost | Lower | Higher |
| Accuracy | Moderate precision | High precision |
| Use Case | Routine measurements | Research and high-end analysis |
Last reviewed on .
Article history
- Latest version
Cite this page:
- Posted by Dayyal Dungrela