Examination of Cerebrospinal Fluid (CSF)
Discover CSF composition, meningitis tests, and neurological conditions. Explore cerebrospinal fluid (CSF) analysis methods, including protein electrophoresis, IgG and albumin ratios, and critical diagnostic values.

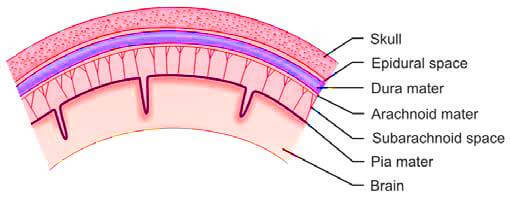
Cerebrospinal fluid (CSF) is a clear, colorless liquid that is produced within the ventricles of the brain, primarily by the choroid plexus, a specialized network of small blood vessels located within the lateral, third, and fourth ventricles. This fluid is mainly an ultrafiltrate of plasma and plays a crucial role in cushioning and protecting the brain and spinal cord. CSF circulates through the cerebral ventricles, the spinal canal, and the subarachnoid space, which is the area between the arachnoid membrane and the pia mater, both of which envelop the brain and spinal cord (Figure 1). The reabsorption of cerebrospinal fluid into the bloodstream occurs through the arachnoid villi, which are small protrusions of the arachnoid membrane that project into the dural venous sinuses.
Composition of Normal Cerebrospinal Fluid in Adults
- Total Volume: 100-150 ml (10-60 ml in newborns)
- Color: Colorless
- Appearance: Clear
- Clot Presence: Absent
- Viscosity: Comparable to water
- Opening Pressure: 60-180 mm of water (10-100 mm in infants and young children)
- Cells:
- Adults: 0-5 cells/cmm
- Infants: 0-30 cells/cmm
- Ages 1-4: 0-20 cells/cmm
- Ages 5-18: 0-10 cells/cmm
- Glucose: 45-80 mg/dl (Typically, CSF glucose is 60% or two-thirds of blood glucose levels)
- Proteins: 15-45 mg/dl (CSF proteins generally constitute 1% of plasma proteins)
- Bilirubin: Absent
- Chloride: 120-130 mEq/L (20 mEq/L higher than serum levels)
- Oligoclonal Bands: Negative
Functions of Cerebrospinal Fluid
- Protection: Acts as a shock absorber, safeguarding the brain and spinal cord from physical injury.
- Nutrient Transport: Serves as an intermediary between the blood and the brain, facilitating the supply of nutrients and the removal of waste products.
Collection of Cerebrospinal Fluid
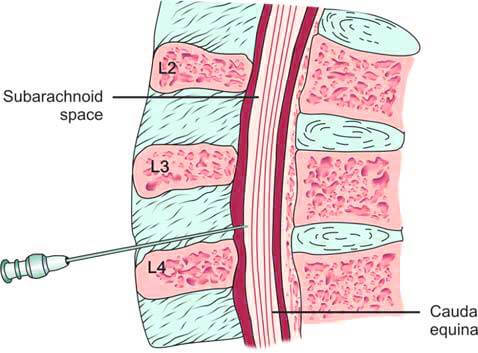
Certain diseases cause distinct changes in cerebrospinal fluid composition, making CSF analysis crucial for diagnosis. The first lumbar puncture (LP) was performed by Quincke in 1891 to obtain a CSF sample. During this procedure, a spinal or LP needle is inserted into the subarachnoid space between the 3rd and 4th, or 4th and 5th lumbar vertebrae (L3-L4 or L4-L5). This location is chosen to avoid damaging the spinal cord, which terminates around the T12 vertebra, below which the cauda equina or nerve roots are located (Figure 2).
The patient assumes a lateral recumbent position, lying on their side with their back perfectly vertical and positioned at the edge of the bed. The knees are drawn up towards the chest, and the head is flexed downwards, resting on the chest. This posture helps to widen the space between the lumbar vertebrae. Alternatively, the patient can also be positioned in a seated posture.
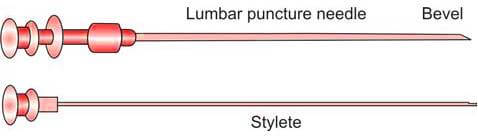
The chosen site for the lumbar puncture is then disinfected using a solution containing chlorhexidine or povidone-iodine. Sterile drapes are applied to maintain a sterile field. Following the administration of a local anesthetic, a sterile lumbar puncture needle, preferably 22 gauge (Figure 3), is carefully and gradually inserted. As the needle advances through the spinal ligaments and dura mater, increased resistance is noted. Upon reaching the subarachnoid space, a sudden decrease in resistance or a ‘give’ is felt. At this point, the stylet is slowly withdrawn. Once cerebrospinal fluid (CSF) begins to appear, a pre-assembled manometer is attached to the needle to measure the opening pressure of the CSF. It is important that the patient remains in the lateral recumbent position during this measurement to ensure accurate results.
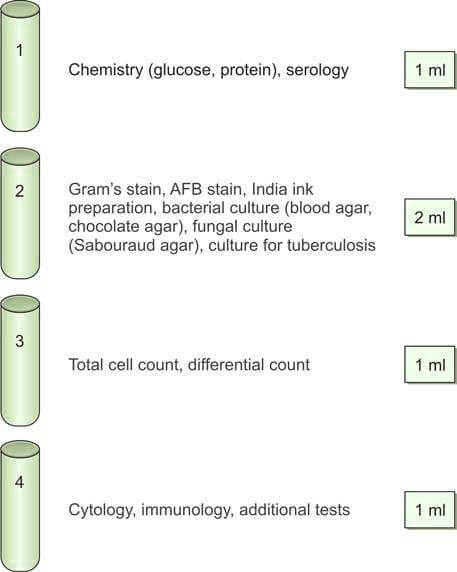
Using a three-way stopcock in the correct position, cerebrospinal fluid (CSF) is collected into sterile plain tubes, as outlined below (Figure 4):
- Tube 1: Chemistry (for glucose, protein, and other serological tests)
- Tube 2: Microbiology (for Gram staining, bacterial culture, and sensitivity testing)
- Tube 3: Hematology (for total cell count and differential count)
- Tube 4: Cytology and special studies
Typically, 3-5 ml of cerebrospinal fluid (CSF) is collected during the procedure. If the initial pressure reading was elevated, the closing pressure is measured after the CSF collection.
Once the CSF has been collected, the needle is carefully withdrawn after the stylet is reinserted. A sterile dressing is then applied to the puncture site. Additionally, a venous blood sample is taken to measure blood glucose levels.
Indications for Lumbar Puncture
- Diagnostic Examination of CSF:
- Suspected meningeal involvement due to leukemia or malignancy.
- Central nervous system (CNS) infections, particularly meningitis (inflammation of leptomeninges) and encephalitis.
- Subarachnoid hemorrhage, particularly when a CT scan is unavailable.
- Inflammatory conditions such as Guillain-Barré syndrome and multiple sclerosis (for diagnostic gammaglobulin analysis).
- Neoplasms of the CNS.
- Indications for emergency lumbar puncture are detailed in Box 1.
- Administration of Medications:
- Antibiotics (e.g., amphotericin B for fungal meningitis).
- Anesthetic agents.
- Anticancer drugs (e.g., methotrexate for acute lymphoblastic leukemia).
- Reduction of CSF Pressure: Treatment of benign intracranial hypertension (pseudotumor cerebri).
- Introduction of Radiographic Contrast Media: Used in myelography for diagnostic imaging.
- Queckenstedt’s Test:
- Employed when normal opening CSF pressure is observed, and there is clinical suspicion of subarachnoid block, tumor, or sinus thrombosis. This test is contraindicated if CSF pressure is elevated due to the risk of precipitating brain herniation.
- The test examines the correlation between CSF pressure and jugular vein pressure (due to continuity with dural venous sinuses where arachnoid villi project). Both jugular veins are compressed and released, with CSF pressure changes observed. Normally, CSF pressure rises rapidly with compression and falls with release. In spinal block cases, the pressure rise is absent, low, or delayed, indicating a positive test in approximately 80% of patients with cord compression. However, with the advancement of myelography, Queckenstedt’s test is rarely performed.
Box 1: Indications for emergency lumbar puncture.
- Suspected meningitis
- Suspected subarachnoid hemorrhage
- Suspected meningeal involvement by leukemia
Complications of Lumbar Puncture
- Post-puncture Headache: This is the most frequent complication, caused by CSF leakage at the puncture site exceeding the rate of CSF production. The risk of headache is higher with the use of a larger bore needle. Utilizing a smaller bore needle (22 gauge) and keeping the patient flat for 2-3 hours post-procedure can mitigate this risk.
- Infection: The introduction of infection into the spinal canal can occur if aseptic techniques are not strictly followed, if septicemia is present, or if there is an infection at the puncture site.
- Subdural Hematoma: Patients with bleeding disorders may develop a subdural hematoma, leading to neurological deficits.
- Failure to Obtain CSF (Dry Tap): This can result from improper patient positioning or an existing spinal block.
- Brain Herniation: Performing a lumbar puncture in patients with high intracranial pressure can cause brain herniation through the tentorium (uncus of the temporal lobe) or foramen magnum (cerebellar tonsils), potentially damaging the brainstem. This risk has led to a decrease in the use of lumbar punctures in recent years.
- Subarachnoidal Epidermal Cyst: Traumatic implantation of a skin plug into the subarachnoid space can lead to the development of an epidermal cyst years after the procedure, particularly if the lumbar puncture is performed without a stylet.
Contraindications to Lumbar Puncture
- Raised Intracranial Pressure: This is often due to a space-occupying lesion such as a brain abscess, posterior fossa tumor, subdural hematoma, or epidural abscess. Patients typically exhibit symptoms including headache, altered pupillary response, absent Doll’s eye reflex, abnormal respiratory patterns, papilledema, bradycardia, hypertension, and decerebrate or decorticate posturing. In such cases, lumbar puncture can lead to brain herniation. If a mass lesion is suspected, cranial CT or MRI should be performed first.
- Cardiorespiratory Compromise: Lumbar puncture is contraindicated in patients with compromised cardiorespiratory function.
- Uncorrected Bleeding Diathesis: Patients with bleeding disorders that have not been corrected are at risk.
- Local Infection: Performing a lumbar puncture at a site with a local infection is contraindicated.
Laboratory Examination of Cerebrospinal Fluid
Transportation and Immediate Examination
After collection, cerebrospinal fluid (CSF) specimens must be transported to the laboratory promptly and examined without delay. This urgency is due to the rapid disintegration of cells and the reduction of glucose levels caused by glycolysis. Ideally, CSF should be analyzed within one hour of collection, with cell counts performed within 30-60 minutes. Using glass tubes for collection is discouraged as cells adhere to glass, leading to reduced cell counts. Additionally, specimens for bacterial culture should not be refrigerated since certain fastidious organisms, such as Haemophilus influenzae and Neisseria meningitidis, do not survive in cold temperatures. CSF chemical examination results should always be compared with plasma levels, as changes in plasma are reflected in CSF.
Examination Components
The comprehensive examination of CSF includes:
- Opening Pressure
- Appearance
- Total and Differential Cell Counts
- Chemical Examination
- Microbiological Examination
- Special Investigations
Opening Pressure
To measure the CSF pressure, attach a manometer to the spinal needle hub. Gently extend the patient’s legs and return the neck to a neutral position. CSF pressure correlates with jugular and vertebral venous pressures. The patient should remain relaxed to avoid artificially altering the pressure; straining or breath-holding increases CSF pressure, while hyperventilation decreases it.
Normal Opening Pressure
- Adults: 60-180 mm of water in the lateral recumbent position.
- Children (under 8 years): 10-100 mm of water.
Causes of Increased CSF Pressure
- Tension and anxiety
- Intracranial mass lesions (e.g., neoplasm, abscess, hemorrhage)
- Meningitis
- Cerebral edema
- Subarachnoid hemorrhage
- Congestive cardiac failure
- Benign intracranial hypertension (pseudotumor cerebri)
Causes of Decreased CSF Pressure
- CSF leakage following trauma or previous lumbar puncture
- Complete spinal block (due to tumor, abscess, adhesions, or herniated intervertebral disk)
A significant difference between opening and closing pressures often indicates a partial or complete spinal block. If the opening CSF pressure exceeds 200 mm, no more than 1-2 ml of CSF should be removed.
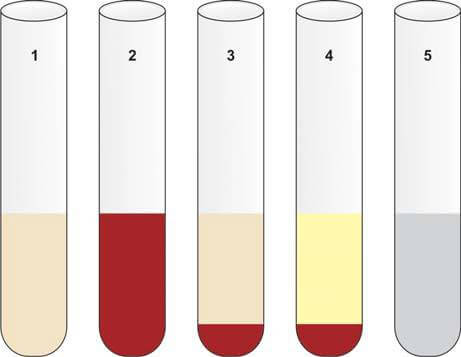
Gross Appearance of Cerebrospinal Fluid
Normal CSF is clear, colorless, and does not clot. Abnormal CSF may appear turbid, blood-mixed, xanthochromic, or viscous (Figure 5). Clot formation in CSF is abnormal and suggests increased protein levels.
Abnormalities in CSF Appearance
- Turbid CSF:
- Leukocytes >200 cells/cmm
- Red cells >400 cells/cmm
- Microorganisms (bacteria, fungi, amebae)
- Radiographic contrast media
- Aspiration of epidural fat during lumbar puncture
- Raised protein levels
- Blood-Mixed CSF: Blood-stained CSF may result from a traumatic tap or subarachnoid hemorrhage. Distinguishing between these is crucial, with traumatic tap involving injury to the venous plexus in the spinal wall. Differences between the two are given in Table 1.
- Xanthochromia: Xanthochromia refers to the yellow discoloration of cerebrospinal fluid (CSF). To detect this, the CSF is centrifuged, and the supernatant is compared with a tube of distilled water of the same size. The primary causes of xanthochromia include:
- Subarachnoid Hemorrhage (SAH): This condition typically occurs about 12 hours after a bleeding episode, often due to the rupture of a cerebral aneurysm. Patients with SAH usually present with a sudden, severe headache in the occipital region, which may be followed by a loss of consciousness. In this condition, red blood cells in the CSF undergo hemolysis, releasing oxyhemoglobin. Within a week, macrophages and other cells in the leptomeninges convert oxyhemoglobin to bilirubin, resulting in the yellow discoloration of the CSF supernatant (Figure 5). The preferred diagnostic tool for subarachnoid hemorrhage is a CT scan to detect blood in the basal cisterns. If the CT scan is negative or inconclusive and xanthochromia is not visibly apparent, a spectroscopic examination of the CSF can confirm the diagnosis by revealing absorption peaks of oxyhemoglobin and bilirubin.
- Jaundice: When serum bilirubin levels exceed 6.0 mg/dl, jaundice can cause xanthochromia in the CSF.
- Elevated CSF Protein Levels: Xanthochromia can also occur when CSF protein levels exceed 150 mg/dl. Additionally, Froin’s syndrome, characterized by a combination of xanthochromia, elevated protein levels in the CSF, and spontaneous clot formation upon standing, results from a complete block of the subarachnoid space.
- Other Abnormal CSF Colors:
- Pink: Hemoglobin breakdown from red cell lysis.
- Brownish: Indicative of meningeal metastatic melanoma.
- Orange: High carotene ingestion.
- Clot Formation: Presence of pellicle or clot formation suggests increased proteins (>150 mg/dl) and occurs in tuberculous meningitis, purulent meningitis, spinal block, and traumatic lumbar puncture. Clotting does not occur in subarachnoid hemorrhage.
- Thick Viscous CSF: Seen in cryptococcal meningitis, meningeal metastatic mucinous adenocarcinoma, severe meningitis, and when nucleus pulposus fluid is released into the CSF due to needle injury to the intervertebral disk.
| Cerebrospinal fluid finding | Traumatic lumbar puncture | Subarachnoid hemorrhage |
|---|---|---|
| Gross appearance | Blood more in initial tubes as compared to later tubes; Blood clots on standing | Blood uniform in all tubes; Blood does not clot on standing |
| Supernatant after centrifugation within 1 hour of collection | Clear | Pink or yellow (xanthochromia); yellow xanthochromia develops 12 hours after hemorrhage |
| Microscopy | Progressive decrease of red cell counts in later tubes | Red cell counts uniform in all tubes; Hemosiderin-laden macrophages present |
| Latex agglutination test for D-dimer* | Negative | Positive |
| Cerebrospinal fluid pressure | Normal | Increased |
| Cerebrospinal fluid protein | Normal | Increased |
| *D-dimer: A cross-linked fibrin degradation product | ||
Cell Counts in Cerebrospinal Fluid
Total Leukocyte Count
Cell counting in cerebrospinal fluid (CSF) is performed manually on an undiluted sample using a counting chamber. This analysis is crucial for diagnosing various disorders, providing essential diagnostic insights when combined with a differential count. An elevated cell count in CSF is termed pleocytosis.
Microscopic examination of all CSF samples is imperative due to the rapid disintegration of cells and their adherence to glass tube walls. CSF samples should ideally be examined promptly after collection to ensure accurate results.
Key Considerations
- Sampling Tube: Use CSF collected in tube 3 for accurate cell counting.
- Dilution: Typically, CSF does not require dilution. Only cloudy samples indicating increased leukocytes may necessitate dilution using a Turk solution (composition: glacial acetic acid 4 ml, methylene blue solution 10 drops, and distilled water to 200 ml) in a 1:20 ratio.
Method
- Preparation: Ensure thorough mixing of the CSF sample. If clear, do not dilute; if cloudy, perform a 1:20 dilution with Turk solution.
- Counting: Cover the counting chamber with the provided coverslip.
- Settling: Allow the fluid to settle for 2 minutes in the counting chamber.
- Chamber Selection: Prefer the Fuchs-Rosenthal counting chamber due to its greater depth compared to the improved Neubauer chamber.
- Counting Process: Count cells in 5 large squares (including 4 corner squares and one central square). For undiluted CSF, the total number of cells in these 5 squares represents the count per cubic millimeter (cmm) of CSF. If diluted, multiply the cell count by the dilution factor (e.g., 20).
Causes of Increased Cell Count in Cerebrospinal Fluid (CSF)
- Meningitis and Other Infections of the Central Nervous System (CNS): Infections in the CNS often lead to an elevated cell count in the CSF, as the immune system responds to the presence of pathogens.
- Intracranial Hemorrhage: Bleeding within the brain can increase the number of cells in the CSF due to the presence of blood.
- Meningeal Infiltration by Malignancy: Cancer cells infiltrating the meninges can result in a higher cell count in the CSF.
- Repeated Lumbar Punctures: Frequent lumbar punctures can cause a transient increase in the CSF cell count.
- Injection of Foreign Substances: Introducing substances such as radiographic contrast media or certain drugs into the subarachnoid space can lead to increased cell counts.
- Multiple Sclerosis: This autoimmune disorder can cause elevated cell counts in the CSF as the body’s immune response targets the CNS.
The presence of blood in the CSF, due to either a traumatic tap or subarachnoid hemorrhage, can artificially raise the leukocyte count by approximately 1 white blood cell (WBC) per 1,000 red blood cells (RBCs). This correction factor should be applied if the patient’s hemogram is normal. In cases of significant anemia or leukocytosis, the leukocyte count in the CSF should be adjusted using the following formula:
Corrected WBC Count in CSF
The corrected WBC count in cerebrospinal fluid (CSF) can be calculated using the following formula:
Corrected WBC Count in CSF = WBC Count in CSF (cells/cmm) - [ ( WBC Count in Blood × Red Cell Count in CSF ) ÷ Red Cell Count in Blood ]
This formula helps in determining the accurate WBC count by accounting for the blood contamination in the CSF sample.
Differential Leukocyte Count (DLC)
The differential leukocyte count provides information on the relative proportions of different leukocytes in the CSF. This count is essential for diagnosing and understanding various conditions affecting the CNS.
- Low Cell Count: If the CSF sample contains few cells, it should be centrifuged at high speed (3000 g) for 10 minutes, and a smear should be made from the sediment.
- High Cell Count: If the CSF sample has a high cell count, a smear can be made directly from the uncentrifuged sample.
After preparing the smear, it should be stained with a Romanowsky stain and examined under a microscope (Figure 6). Simple centrifugation often causes cell breakage and distortion. Therefore, using a cytospin preparation with a cytocentrifuge, which concentrates cells into a uniform monolayer on a slide, is recommended. This method improves cell yield and preserves cell morphology.
In normal adults, the differential count typically shows approximately 70% lymphocytes and 30% monocytes. In young children, a higher proportion of monocytes (up to 70%) is common. The causes of increases in different types of leukocytes in the CSF are detailed in Table 2.
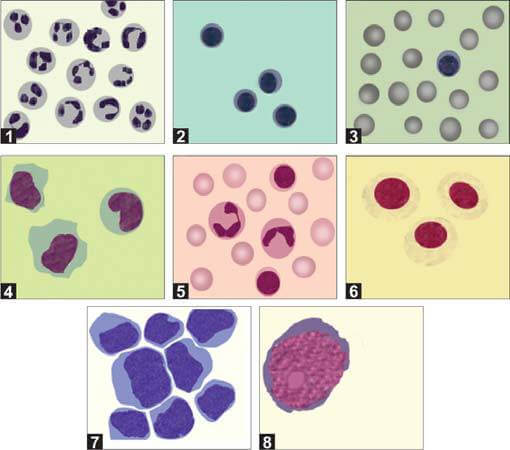
| Predominant neutrophils | Predominant lymphocytes | Mixed cell pattern (neutrophils, lymphocytes, monocytes) | Predominant eosinophils |
|---|---|---|---|
|
|
|
|
Other Cells in Cerebrospinal Fluid (CSF)
Cerebrospinal fluid (CSF) may contain cells other than mature blood cells, such as immature hematopoietic cells, tissue cells (including ependymal cells and pia-arachnoid mesothelial cells), and malignant cells. CSF analysis is particularly significant in cases of acute lymphoblastic leukemia (ALL) to ascertain CNS involvement. An increased white blood cell (WBC) count greater than 5 cells/μL, accompanied by the presence of lymphoblasts, indicates CNS involvement in leukemia.
Chemical Examination of Cerebrospinal Fluid
The routine chemical examination of CSF involves the estimation of protein and glucose levels. For these tests, CSF from tube 1 is typically used.
Estimation of Proteins in CSF
The normal protein level in CSF for adults ranges from 15 to 45 mg/dL. An elevated protein level in the CSF is a sensitive, albeit non-specific, indicator of CNS pathology. It is noteworthy that CSF protein levels may remain normal during the early stages of meningitis, with significant elevations (greater than 150 mg/dL) occurring in cases of bacterial meningitis.
Several methods are employed for the estimation of CSF proteins, with the turbidimetric method using trichloroacetic acid being widely used. In this method, the addition of trichloroacetic acid to CSF leads to protein precipitation, resulting in a turbid solution. The degree of turbidity is then compared to a standard protein concentration using a photoelectric colorimeter.
In instances where a lumbar puncture results in blood contamination (traumatic tap), the protein levels may be falsely elevated. To correct this, 1 mg/dL of protein should be subtracted for every 1,000 red blood cells per cubic millimeter (cmm) present in the sample. For this correction to be accurate, the red cell count and protein levels should be measured from the same tube of CSF.
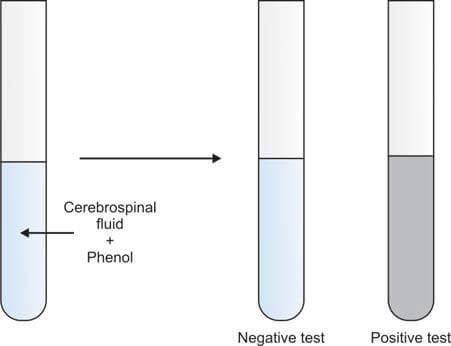
If laboratory facilities do not allow for the estimation of CSF proteins, Pandy’s test for globulins may be performed. In this test, CSF is added to a saturated solution of phenol. The immediate development of cloudiness indicates the presence of increased globulins, and the test is reported as positive. If no cloudiness appears, the test is reported as negative (Figure 7).
In addition to measuring cerebrospinal fluid (CSF) proteins, it is crucial to concurrently assess serum proteins for accurate interpretation of results.
Conditions Associated with Elevated CSF Proteins
Elevated protein levels in CSF can indicate various underlying conditions, which include:
- Increased Capillary Permeability of the Blood-Brain Barrier: Conditions such as meningitis lead to heightened capillary permeability, allowing proteins to leak into the CSF.
- Mechanical Obstruction to CSF Circulation: Disorders such as spinal cord tumors cause mechanical obstructions, resulting in stasis and increased fluid reabsorption, thereby raising protein levels in the CSF.
- Enhanced Local (Intrathecal) Immunoglobulin (IgG) Production: Diseases such as multiple sclerosis, neurosyphilis, and subacute sclerosing panencephalitis stimulate local prod
- Combination of Increased Capillary Permeability and Local IgG Production: Conditions like Guillain-Barré syndrome exhibit both elevated capillary permeability and enhanced local IgG production.
- Hemorrhage in the CSF: Instances of traumatic tap and subarachnoid hemorrhage introduce blood into the CSF, artificially increasing protein levels.
Marked elevations in CSF protein levels (greater than 500 mg/dL) are observed in cases of complete spinal block due to tumors, bacterial meningitis, and bloody CSF.
Differential Diagnosis of Elevated CSF Proteins
The differential diagnosis of elevated CSF proteins—distinguishing between increased capillary permeability of the blood-brain barrier and increased intrathecal synthesis of immunoglobulin G (IgG)—can be determined using specific parameters:
- CSF Albumin/Serum Albumin Ratio: Albumin is neither synthesized nor metabolized in the CNS. Therefore, an increased CSF albumin/serum albumin ratio signifies elevated permeability of the blood-brain barrier.
- CSF IgG/Serum IgG Ratio: IgG can be synthesized within the CNS. An increased CSF IgG/serum IgG ratio may indicate either enhanced permeability of the blood-brain barrier or increased intrathecal synthesis of IgG.
- CSF IgG/Albumin Index: This index specifically indicates local IgG production within the CNS, differentiating it from increased permeability issues.
The differential diagnosis of elevated protein levels in cerebrospinal fluid (CSF), distinguishing between increased capillary permeability of the blood-brain barrier and increased intrathecal synthesis of immunoglobulin G (IgG), can be determined using specific diagnostic parameters, as detailed in Table 3.
| CSF/Serum albumin ratio | CSF/Serum IgG ratio | CSF IgG/Albumin index | Causes |
|---|---|---|---|
| Increased | Increased | Normal | Increased permeability of blood-brain barrier |
| Normal | Increased | Increased | Increased intrathecal synthesis of proteins |
Estimation of Glucose in Cerebrospinal Fluid (CSF)
The normal glucose level in CSF is approximately two-thirds of the blood glucose level, with a typical CSF to blood glucose ratio of 0.6. For accurate comparison, a blood glucose sample should be obtained one hour prior to the lumbar puncture (LP). Immediate processing of the CSF sample is essential to prevent falsely low glucose results due to glycolysis.
CSF glucose levels are commonly measured using the glucose oxidase method, with a normal range of 45-80 mg/dl. A CSF glucose level below 40 mg/dl is considered abnormal.
Decreased CSF glucose levels can result from the utilization of glucose by bacteria (either pyogenic or tuberculous), leukocytes, or cancer cells within the CSF. Conditions associated with decreased CSF glucose include:
- Acute bacterial meningitis
- Tuberculous meningitis
- Fungal meningitis
- Meningeal involvement by malignant tumors (meningeal carcinomatosis)
- Hypoglycemia
In cases of viral meningitis, CSF glucose levels typically remain normal.
Microbiological Examination Cerebrospinal Fluid (CSF)
Various microbiological tests can be performed on CSF samples to diagnose infections:
- Direct Wet Mount of CSF: Used in suspected cases of cryptococcosis, amebic meningoencephalitis, Candida infection, and trypanosomiasis.
- Gram’s Stain: Recommended if the CSF is turbid and neutrophils are elevated.
- Ziehl-Neelsen Stain: Utilized when tuberculous meningitis is suspected.
- Latex Agglutination Tests: For the detection of bacterial and cryptococcal antigens.
- Serological Tests: Conducted for syphilis.
- Culture: For the identification of bacteria and Mycobacterium tuberculosis.
- Polymerase Chain Reaction (PCR): Used to detect Mycobacterium tuberculosis and various viruses.
Direct Wet Mount of CSF
To perform this test, a drop of CSF sediment (obtained through centrifugation) is placed on a glass slide, covered with a cover slip, and examined under a microscope with reduced illumination. Look for motile trypanosomes or sluggishly moving amebae such as Naegleria fowleri. This free-living ameba, found in water, can enter the body through the nose and travel to the central nervous system, causing a fatal hemorrhagic meningoencephalitis. Candida albicans may be observed as oval budding forms and pseudohyphae in an unstained wet mount.
If cryptococcal meningitis is suspected, the wet mount should be examined using dark-field microscopy. Alternatively, a drop of India ink can be added to the sediment on a slide, covered with a cover slip, and examined under a microscope at ×40 magnification. Cryptococcus neoformans will appear as spherical, budding yeast forms, 2-10 μm in diameter, surrounded by a large unstained capsule (Figure 8). The India ink preparation can detect cryptococci in approximately 50% of cryptococcal meningitis cases.
Gram’s Smear
Gram’s staining is essential when the cerebrospinal fluid (CSF) appears purulent and there is an increase in neutrophils. The Gram stain can detect bacteria in 80% of untreated bacterial meningitis cases and in 60% of cases that have been partially treated. Consequently, a negative Gram stain result does not definitively exclude the presence of a bacterial infection.
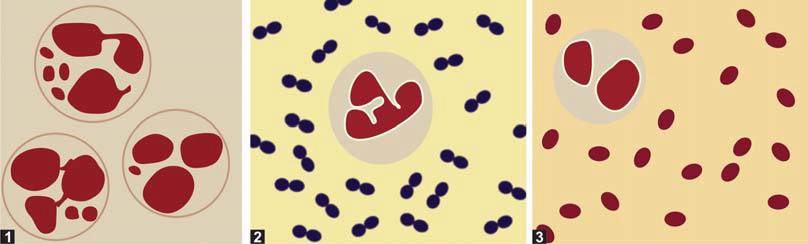
Cerebrospinal fluid (CSF) is centrifuged, and a smear of the sediment is prepared on a glass slide. If the CSF appears purulent, the smear is made directly without prior centrifugation. The smear is then air-dried, stained using Gram’s method, and examined under an oil-immersion lens to detect bacteria (Figure 9). The bacteria commonly responsible for meningitis include:
- Neisseria meningitidis (Meningococci): Gram-negative diplococci found inside neutrophils.
- Streptococcus pneumoniae (Pneumococci): Gram-positive diplococci surrounded by an unstained capsule.
- Haemophilus influenzae: Gram-negative coccobacilli.
- Escherichia coli: Gram-negative rods.
In cases of meningitis caused by these bacteria, the accompanying cells are typically polymorphonuclear neutrophils.
The detection of characteristic bacteria through Gram staining can indicate the causative agent of meningitis. However, a definitive diagnosis necessitates CSF culture.
There is a correlation between the patient’s age and the causative organism of meningitis:
- 0 to 6 months: Group B streptococci, Escherichia coli, Listeria monocytogenes
- 6 months to 6 years: Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae type B, Enteroviruses
- 6 to 60 years: Neisseria meningitidis, Streptococcus pneumoniae, Enteroviruses, herpes simplex virus
- >60 years: Streptococcus pneumoniae, gram-negative bacilli, Listeria monocytogenes
Ziehl-Neelsen Staining for Mycobacterium tuberculosis
In cases of tuberculous meningitis, the concentration of tubercle bacilli in cerebrospinal fluid (CSF) is generally low, making Ziehl-Neelsen or acid-fast bacillus (AFB) staining a less sensitive method for detecting M. tuberculosis. AFB smears return negative results in approximately 70% of tuberculous meningitis cases. Fluorescent auramine staining offers better sensitivity compared to Ziehl-Neelsen staining.
Latex Agglutination Tests
Commercially available latex agglutination tests for bacterial antigens are noted for their sensitivity, rapidity, and ease of use. These tests can detect N. meningitidis (groups A, B, C, Y, and W135), H. influenzae (capsular type B), S. pneumoniae, and S. agalactiae. However, these tests are costly, and their sensitivity is comparable to that of Gram’s smear. Due to the potential for false-positive results, they are not recommended for routine meningitis diagnosis but are particularly useful in patients who have received partial treatment and have negative Gram’s stain and culture results.
Latex agglutination tests for cryptococcal antigens are also available, boasting a sensitivity of 90%. These tests have largely supplanted India ink preparations for diagnosing cryptococcal meningitis.
Limulus Lysate Assay for Endotoxin Produced by Gram-negative Bacteria
The Limulus amebocyte lysate assay is a rapid, sensitive, and specific test for detecting endotoxins produced by gram-negative bacteria such as N. meningitidis, H. influenzae type b, E. coli, and Pseudomonas. This assay is particularly useful for rapidly diagnosing infections in newborns, where these pathogens are prevalent.
Serologic Tests for Syphilis
When neurosyphilis is suspected, and the fluorescent treponemal antibody absorption (FTA-ABS) test returns positive in serum, the Venereal Disease Research Laboratory (VDRL) test should be performed on the CSF. While the VDRL test is highly specific, it lacks sensitivity; thus, a positive result confirms neurosyphilis, but a negative result does not exclude it. Other serological tests for syphilis are not suitable for diagnosing neurosyphilis in the CSF.
A combination of a positive FTA-ABS test in serum and a reactive VDRL test in CSF is diagnostic of active neurosyphilis.
CSF Culture
CSF culture is recommended when bacteria are observed on a Gram-stained smear or when there is an increase in leukocytes or protein levels. It remains the gold standard for diagnosing bacterial meningitis. The CSF sample collected in tube 2 is typically used for culture. The sensitivity of CSF culture for identifying bacteria is approximately 90%, though it decreases in partially treated cases. For culture, CSF is generally inoculated on chocolate (heated blood) agar and blood agar. In newborns, the sample is also inoculated on McConkey’s agar.
In cases of tuberculous meningitis, culture for Mycobacterium tuberculosis is positive in about 56% of cases. The sensitivity of detection increases when a larger volume of CSF (e.g., 10 ml) is used for inoculation.
In cryptococcal meningitis, the culture is positive in 95% of cases.
Polymerase Chain Reaction (PCR)
PCR is a highly specific and sensitive method for diagnosing CNS infections. It employs probes to detect genes specific to the infecting organism. This test is rapid and requires only a small amount of CSF. However, its high cost and limited availability to specialist laboratories are significant drawbacks. CSF PCR is particularly useful for diagnosing viral CNS infections (e.g., herpes simplex, enteroviruses, varicella-zoster) and tuberculous meningitis. CSF findings in various types of meningitis are summarized in Table 4.
| Condition | Appearance | Leukocytes | Protein in mg/dl | Glucose in mg/dl | Additional investigations |
|---|---|---|---|---|---|
| Normal | Clear, colorless | <5/μl (mostly lymphocytes) | 15-45 | 45-80 | - |
| Acute pyogenic meningitis | Turbid or purulent | Increased (>1000/μl); mostly neutrophils | Increased; 50-1500 | Decreased; <40 | Gram’s stain; culture; Latex agglutination test |
| Tuberculous meningitis | Clear or cloudy | Increased (100-600/μl); mostly lymphocytes or both lymphocytes and neutrophils | Increased; 45-300 | Decreased; 10-45 | AFB stain; culture; polymerase chain reaction |
| Viral meningitis | Clear or cloudy | Increased (6-300/μl); lymphocytes | Increased | Normal | polymerase chain reaction |
Special Investigations
CSF Protein Electrophoresis
Protein electrophoresis of normal cerebrospinal fluid (CSF) differs from serum primarily due to the presence of a distinct transthyretin band and an additional transferrin band known as β₂-transferrin or tau protein. This specialized examination serves two primary purposes:
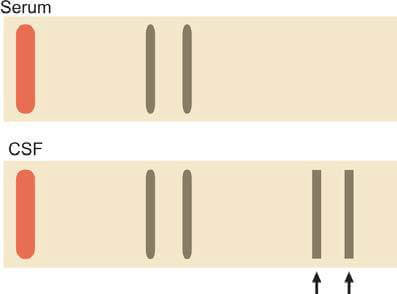
- Identification of Oligoclonal Bands: Agarose gel electrophoresis of concentrated CSF is utilized to detect oligoclonal bands, which appear as two or more discrete bands in the gamma region. The presence of oligoclonal bands in CSF, absent in concurrently analyzed serum protein electrophoresis, indicates intrathecal synthesis of immunoglobulins (Figure 10). While commonly found in multiple sclerosis (present in 90% of patients), oligoclonal bands are not exclusive to this condition and can also be observed in subacute sclerosing panencephalitis, viral CNS infections, neurosyphilis, and Guillain-Barré syndrome.
- Detection of CSF Leakage: Occasionally, clear fluid leaking from the nose or ear post-trauma or surgery requires examination to confirm if it is CSF. Accurate identification is crucial to prevent the risk of recurrent meningitis. Protein electrophoresis with immunofixation for transferrin is recommended for this purpose. CSF protein electrophoresis typically reveals an additional transferrin band, referred to as tau protein, which is enzymatically modified and migrates just behind unaltered transferrin in the β region. This distinctive pattern, with two transferrin isoform bands, is highly sensitive and specific for confirming the fluid as CSF (Figure 11). No other body fluids or secretions exhibit this secondary transferrin isoform band.

Measurement of Albumin and Immunoglobulin G (IgG)
The comparison of the CSF/plasma ratio of Immunoglobulin G (IgG) and albumin can provide valuable diagnostic insights, particularly in conditions like multiple sclerosis where a high ratio indicates intrathecal synthesis of IgG.
Reference Ranges
Reference ranges are given at the beginning of this article under "Composition of Normal Cerebrospinal Fluid in Adults".
Critical Values
- Cells: >10/cmm
- Glucose: <45 mg/dl
- Proteins: >45 mg/dl
- Detection of pathogens on Gram stain, latex agglutination tests, Ziehl-Neelsen stain, or India ink preparation
- Positive culture
- Detection of blast cells or malignant cells
- Positive polymerase chain reaction for herpes simplex
FAQs
How to test for CSF leak at home?
The information on this page is peer reviewed by a qualified editorial review board member. Learn more about us and our editorial process.
Last reviewed on .
Article history
- Latest version
Reference(s)
- Riordan, F A I., et al. “When to do a lumbar puncture.” Archives of disease in childhood, vol. 87, no. 3, 2002, pp. 235-237., doi: 10.1136/adc.87.3.235.
- Seehusen, Dean A., et al. “Cerebrospinal fluid analysis.” Am Fam Physician, vol. 68, no. 3, 2003, pp. 1103-1109., pmid: 14524396.
Cite this page:
- Posted by Dayyal Dungrela