Tests for Detection of Ketones in Urine
Discover methods like Rothera's and Gerhardt's tests for ketone detection in urine. Explore ketonuria, associated with diabetes and starvation.

The examination of ketone bodies in urine is employed to assess the ketone levels in your urine. Typically, the body utilizes sugar as its primary energy source. However, when there is an insufficient supply of sugar for energy, the body shifts to burning fat and generates compounds known as ketones. These ketones find their way into both the bloodstream and urine.
While it is normal to have a minimal quantity of ketones in the body, elevated levels can lead to severe illness or even fatalities.
This test may be necessary if you exhibit elevated blood sugar levels, a condition often associated with diabetes. Individuals with diabetes commonly experience heightened ketone levels due to insufficient insulin production or inadequate responsiveness to insulin, leading to an inability to utilize blood sugar for energy. Regular monitoring of ketone levels becomes imperative for those with elevated blood sugar levels and either type 1 or type 2 diabetes.
However, it's worth noting that individuals without diabetes can also display ketones in their urine, especially when the body relies on fat for energy instead of glucose. This metabolic shift may occur with factors such as chronic vomiting, intense physical exertion, adherence to low-carbohydrate diets (such as ketogenic or keto diets), or in the presence of eating disorders.
Particular attention to ketone levels is necessary if you have diabetes and experience:
- Blood sugar levels surpassing 300 mg/dL
- Alcohol misuse
- Diarrhea
- Cessation of carbohydrate intake, like rice and bread
- Pregnancy
- Periods of fasting
- Episodes of vomiting
- Presence of an infection
Your healthcare provider may prescribe or recommend this test if you manifest symptoms such as frequent urination, increased thirst, muscle aches, fatigue, weight loss, shortness of breath, nausea, vomiting, abdominal pain, confusion, or if there is a detectable fruity smell on your breath. Regular monitoring serves as a proactive measure in managing and maintaining optimal health.
Test Procedure
The concentration of ketone bodies in urine during ketosis can vary: β-hydroxybutyric acid comprises approximately 78%, acetoacetic acid constitutes around 20%, and acetone makes up roughly 2%.
No single method for detecting ketonuria reacts to all three ketone bodies. The Rothera’s nitroprusside method, along with methods derived from it, identifies acetoacetic acid and acetone, with the test demonstrating a sensitivity that is 10-20 times higher for acetoacetic acid compared to acetone. The ferric chloride test exclusively identifies acetoacetic acid. Notably, none of the screening tests are capable of detecting β-hydroxybutyric acid.
The techniques employed for identifying ketone bodies in urine encompass Rothera’s test, the Acetest tablet method, the ferric chloride test, and the reagent strip test.
Method 1: Rothera’s’ Test (Classic Nitroprusside Reaction)
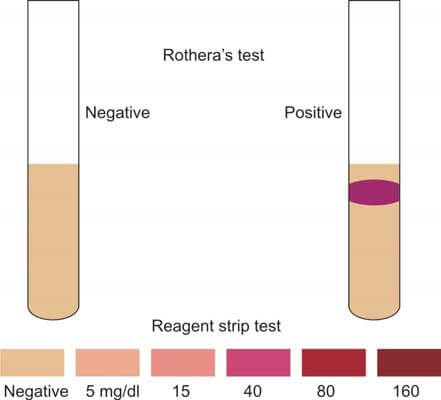
When acetoacetic acid or acetone comes into contact with nitroprusside in an alkaline solution, a distinctive purple-colored complex is produced (refer to Figure 1). Rothera's test exhibits sensitivity in the range of 1-5 mg/dl for acetoacetate and 10-25 mg/dl for acetone.

Method
- Begin by taking 5 ml of urine and placing it into a test tube, then saturate it with ammonium sulfate.
- Introduce a small crystal of sodium nitroprusside and ensure thorough mixing.
- Gradually add liquor ammonia along the side of the test tube to create a layered effect.
- Observe for an immediate formation of a distinct purple permanganate-colored ring at the junction of the two fluids; this signifies a positive test (refer to Figure 2).
It's essential to note that a false-positive result may occur in the presence of L-dopa in urine and in cases of phenylketonuria.
Method 2: A Acetest Tablet Test
Presented here is Rothera's test in tablet form, known as the Acetest tablet. Comprising sodium nitroprusside, glycine, and an alkaline buffer, the tablet exhibits a purple lavender discoloration when acetoacetate or acetone is present (at levels ≥ 5 mg/dl). An approximate quantification of ketone bodies can be derived by comparing the tablet's color with the provided manufacturer's color chart.
It's noteworthy that this test surpasses the reagent strip test in terms of sensitivity for detecting ketones.
Method 3: Ferric Chloride Test (Gerhardt’s)
Upon introducing a 10% ferric chloride solution to urine, the solution undergoes a color change, turning reddish or purplish in the presence of acetoacetic acid. It's crucial to note that the test lacks specificity, as certain drugs, such as salicylate and L-dopa, can yield a similar reaction. The sensitivity of this test is set at a range of 25-50 mg/dl.
Method 4: Reagent Strip Test
Reagent strip tests represent adaptations of the nitroprusside test, as illustrated in Figures 1 and 2. These tests exhibit a sensitivity level of 5-10 mg/dl for acetoacetate. It's important to note that reagent strips can yield false-negative results when exposed to moisture. The ketone pad on the strip test is particularly susceptible to damage due to improper storage.
The information on this page is peer reviewed by a qualified editorial review board member. Learn more about us and our editorial process.
Last reviewed on .
Article history
- Latest version
Cite this page:
- Comment
- Posted by Dayyal Dungrela