Diagnosis of Acute Myelocytic Leukemia (AML) and Acute Lymphocytic Leukemia (ALL)
Discover the signs, symptoms, and diagnostic criteria for acute leukemia. Explore the laboratory findings in acute myelocytic leukemia (AML) and acute lymphocytic leukemia (ALL). Understand the recommended tests for individuals with suspected leukemia.

Leukemia denotes a group of malignant clonal hematopoietic stem cell disorders characterized by the rapid proliferation of blast cells within the bone marrow, often leading to a swiftly progressive and potentially fatal outcome if left untreated. Acute leukemia (AL) constitutes primary bone marrow disorders, commonly referred to as blood cancer.
What is acute leukemia?
Acute leukemia represents a form of malignant clonal hematopoietic stem cell disorder distinguished by the swift escalation in blast cell count within the bone marrow, culminating in a rapidly progressive and potentially fatal course if left unattended. These primary bone marrow disorders, synonymous with blood cancer, fall under the umbrella of acute leukemia (AL).
Classification of Acute Leukemias
The prevailing classification system for acute leukemias is the French-American-British (FAB) Cooperative Group classification, initially introduced in 1976. This classification scheme delineates acute leukemias into two principal types based on morphological and cytochemical criteria:
- Acute Myeloid Leukemia (AML)
- Acute Lymphoblastic Leukemia (ALL)
Each of these types further undergoes subclassification, as depicted in Table 1.
| Acute leukemia | Classification |
|---|---|
| Acute myeloid leukemia (AML) |
|
| Acute lymphoblastic leukemia (ALL) |
|
In contrast to the French-American-British (FAB) classification, the World Health Organization (WHO) classification delineates AML into distinct entities, recognizing AML with recurrent cytogenetic abnormalities, AML with multilineage dysplasia, and therapy-related AML.
Patients harboring clonal, recurrent cytogenetic abnormalities enumerated in Table 2 are classified as having AML regardless of the percentage of blasts present in the blood or bone marrow. These individuals exhibit characteristic clinical and morphological features and typically demonstrate a favorable response to therapy.
| Acute Myeloid Leukemia (AML) | Classification |
|---|---|
| Acute myeloid leukemia with recurrent genetic abnormalities |
|
| Acute myeloid leukemia with multilineage dysplasia |
|
| AML and MDS, therapy related |
|
| AML, not otherwise categorized |
|
Difference between Acute Myelocytic Leukemia (AML) and Acute Lymphocytic Leukemia (ALL)
| Characteristic Features | Acute Myelocytic Leukemia | Acute Lymphocytic Leukemia |
|---|---|---|
| Origin of cells | Myeloid series cells | Lymphoid series cells |
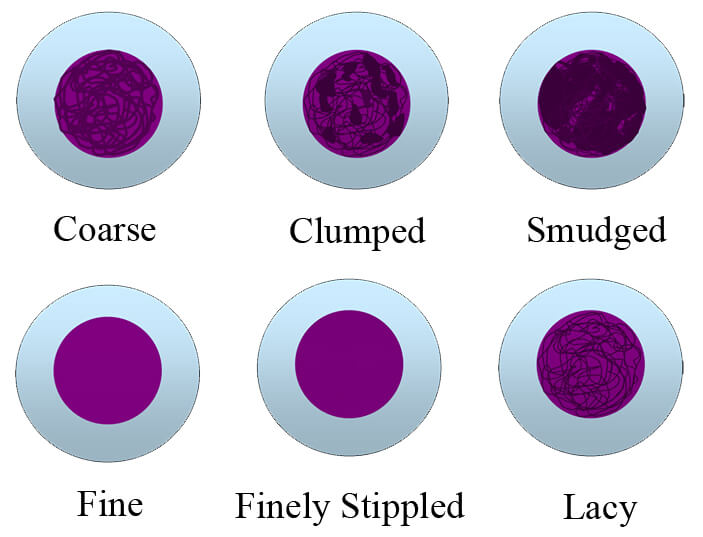
| Characteristics of blast cells | Cell is large in size with moderate cytoplasm. Chromatin patterns are fine and lacy. Nucleoli are prominent and are more than two. | Cell is small with scanty cytoplasm. Chromatin patterns are dense. Nucleoli are indistinct and are less than two. |
| Bone marrow | Mixed population of blast and myeloid cells. | Mainly blast cells and very few WBCs or RBCs |
| Sudan black and peroxidase | Positive | Negative |
| Auer rods | Present | Absent |
| Periodic Acid-Schiff (PAS) | Positive in Erythroblast in M6 leukemia | Positive (block patterns) in L1 and L2, and negative in L3 |
| Leukocyte Alkaline phosphatase (ALP) | Positive (It is used to differentiate CML from the leukemoid reaction) | Negative |
Signs and Symptoms of Acute Leukemia
- Weakness and fatigue
- Low-grade fever
- Bone pain
- Bruising and mild bleeding from gums
- Sudden onset
Diagnosis of Acute Leukemia
- Acute leukemia predominantly affects the younger age group and is more prevalent among children, typically manifesting before the age of 20.
- Anemia develops rapidly, often characterized by normocytic and normochromic red blood cells. Nucleated red blood cells may be observed in the peripheral blood smear.
- Leukocyte (white blood cell) count varies, frequently falling below 100,000/μl.
- Thrombocytopenia is common, accompanied by the presence of petechiae and purpura on the skin and mucous membranes.
Laboratory Findings in Acute Myelocytic Leukemia (AML)
- Total leukocyte count (TLC) exhibits variability, with approximately 25% of patients presenting TLC < 5000/μl, 25% with TLC > 50,000/μl, and 5% to 10% displaying TLC between 5000 to 10,000/μl.
- Abnormal and immature white blood cells are evident.
- Elevated uric acid levels occur in approximately 50% of cases.
- Bone marrow examination typically reveals > 20% blast cells, with numerous promyelocytes, particularly in M3 (acute promyelocytic leukemia).
- Cytochemical stains such as Sudan black and peroxidase demonstrate positive reactions.
- Auer rods may be observed in the cytoplasm of cells, and nucleated red blood cells may also be present.
- Activated partial thromboplastin time (PTT), prothrombin time (PT), and thrombin time are elevated.
- Thrombocytopenia may be present, with platelet counts ranging between 30,000 to 100,000/μl.
Laboratory Findings in Acute Lymphocytic Leukemia (ALL)
- Total leukocyte count varies widely, ranging from low to very high.
- Anemia and thrombocytopenia are common.
- Bone marrow examination reveals a scarcity of normal red and white blood cells, with blast cells being predominant. Sudan black and peroxidase staining show negative reactions, and Auer rods are typically absent.
Test Values for Layman Understanding
Examination of peripheral blood smear and bone marrow is recommended if:
- The patient presents with a high total leukocyte count (TLC).
- The patient exhibits symptoms of anemia.
- Enlarged lymph nodes are observed.
- The patient experiences weakness and fever.
The information on this page is peer reviewed by a qualified editorial review board member. Learn more about us and our editorial process.
Last reviewed on .
Article history
- Latest version
Reference(s)
- Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukemias: French-American-British (FAB) Cooperative Group. Br J Hematol 1976;33:451-8.
- Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia. Ann Intern Med 1985;103:620-5.
- Jaffe ES, Harris NL, Stein H, Vardiman JW (Eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Hematopoietic and Lymphoid Tissues. Lyon; IARC Press, 2001.
- Bennett JM, Catovsky D, Daniel MT, et al. Proposal for the recognition of minimally differentiated acute myeloid leukemia (AML-M0). Br J Hematol 1991; 78:325-9.
Cite this page:
- Comment
- Posted by Dayyal Dungrela