This Giant Bacterium Is So Big You Can See It, and It’s Changing Everything We Know About Life
Scientists have found a giant bacterium visible to the naked eye that stores DNA in tiny compartments, redefining how simple life is organized.
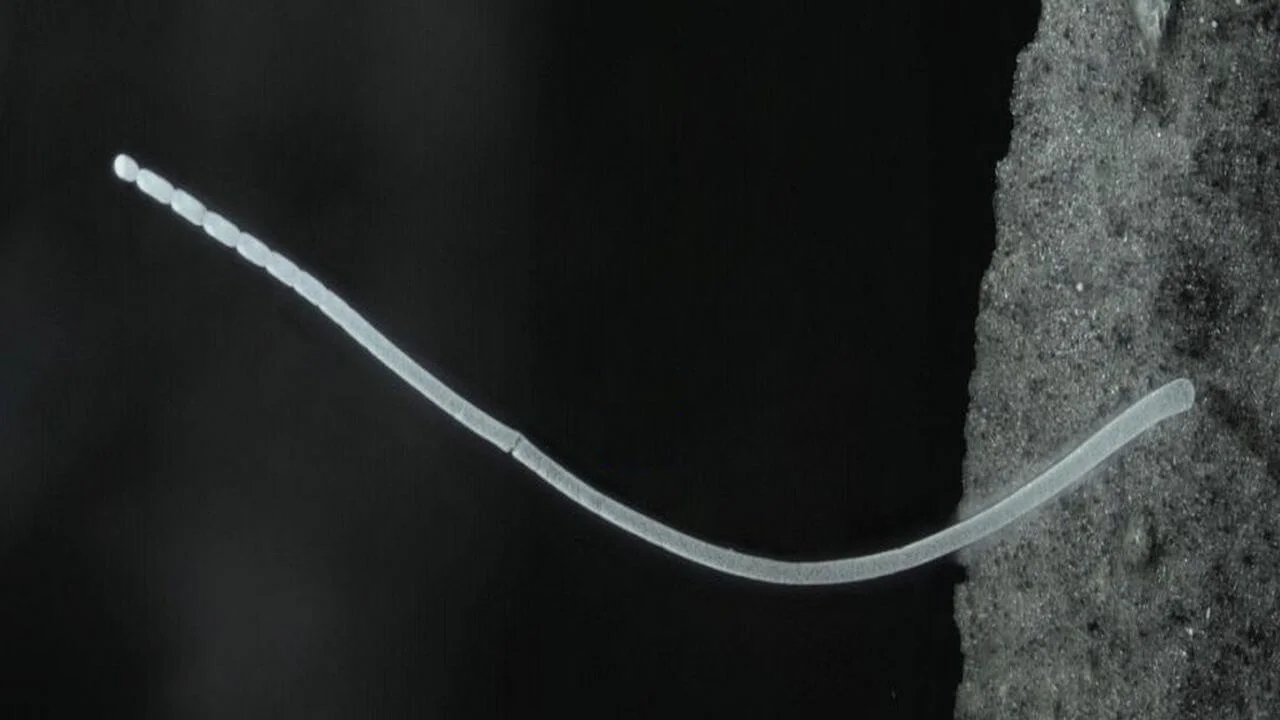
For centuries, biology textbooks have taught that bacteria are microscopic, simple, and structureless. Yet, in a mangrove swamp in Guadeloupe, scientists have found a bacterium that defies every one of those assumptions. The newly discovered Candidatus Thiomargarita magnifica is a single cell that can grow over a centimeter long, making it the largest bacterium ever known and the first visible to the naked eye.
At first glance, the organism looks like a thin white thread clinging to decaying mangrove leaves. But when examined closely, it revealed something astonishing. Instead of being a chain of smaller bacterial cells, it is one enormous cell containing thousands of membrane-bound compartments filled with DNA and ribosomes. These tiny structures, named pepins, are miniature biochemical hubs that perform essential functions once thought impossible for bacteria.
The discovery of Ca. T. magnifica has transformed how scientists define what a bacterium can be. It bridges the conceptual gap between single-celled bacteria and the more complex cells of plants and animals.
A Surprise Hidden in Mangrove Mud
The story began when researchers exploring tropical mangroves noticed unusually large white filaments attached to submerged leaves. Each filament was over 9 millimeters long, with some reaching a full 2 centimeters. At first, scientists assumed the filaments were colonies of many smaller cells, as is common in marine bacteria. However, microscopic and genomic analyses soon revealed that each filament was a single, continuous cell.
Using advanced imaging methods such as confocal microscopy, X-ray tomography, and electron microscopy, researchers mapped the entire structure in remarkable detail. They found that most of the internal space is taken up by a large central vacuole, which pushes the living cytoplasm into a thin outer layer. This design reduces the distance that nutrients and energy molecules must travel, allowing the cell to function despite its massive size.
Inside the Cell: The Hidden World of Pepins
Within this thin layer of cytoplasm lies the bacterium’s most surprising feature—thousands of membrane-bound compartments known as pepins. Each pepin contains both DNA and ribosomes, the machinery responsible for producing proteins. This organization is unique among bacteria, which usually store DNA freely in the cytoplasm without any membrane separation.
In simple terms, these pepins act like microscopic “rooms” inside the cell, each managing its own local operations. The researchers believe this structure helps the cell maintain efficiency despite its large size. By distributing genetic and protein-making functions throughout the cytoplasm, the bacterium avoids the diffusion limitations that usually restrict bacterial growth.
The presence of pepins represents a major evolutionary innovation. It shows that bacteria can develop complex internal structures similar in some ways to the nuclei and organelles found in plant and animal cells.
A Cell Packed with Half a Million Genomes
Beyond its size and structure, Ca. T. magnifica also carries an extraordinary amount of genetic material. Each cell contains hundreds of thousands of DNA copies, making it one of the most polyploid organisms ever discovered. This redundancy may help the bacterium repair DNA damage and regulate metabolism more effectively across its massive body.
The complete genome is enormous by bacterial standards, reaching nearly 12 million base pairs and encoding over 11,000 genes. Many of these genes are linked to energy metabolism and sulfur processing, reflecting the bacterium’s habitat among sulfur-rich mangrove sediments. Others are associated with the production of complex molecules, suggesting potential for discovering new natural compounds with pharmaceutical value.
How It Lives and Grows
Ca. T. magnifica thrives in the unique environment of mangrove swamps, where decaying organic matter provides a steady supply of sulfur and other nutrients. The bacterium oxidizes sulfur for energy, a process common among Thiomargarita species.
Unlike most bacteria that divide evenly to reproduce, this organism grows as a long filament and reproduces by budding small daughter cells from its tip. These small cells can then disperse and start new filaments, a strategy more similar to multicellular development than typical bacterial fission.
Protein labeling experiments confirmed that pepins are metabolically active and capable of producing new proteins, while the membranes scattered throughout the cytoplasm contain enzymes that generate ATP, the cell’s main energy currency. In essence, Ca. T. magnifica has built an internal network that decentralizes its metabolic functions, allowing it to remain active across its vast cellular body.
Why This Discovery Matters
The implications of this discovery reach far beyond the mangrove swamp. It challenges fundamental definitions of what it means to be a bacterium and reshapes the boundaries between simple and complex life forms.
Until now, scientists believed that membrane-bound organelles were exclusive to eukaryotes—the domain that includes animals, plants, and fungi. The presence of pepins in Ca. T. magnifica suggests that complex compartmentalization can also evolve in bacteria, possibly as an adaptation to extreme size or environmental pressures.
From an evolutionary perspective, this discovery provides a glimpse into how cellular complexity may have evolved on Earth. It shows that features resembling eukaryotic organization can arise independently in prokaryotes, offering new clues about the transitional stages that led to complex life.
Moreover, the bacterium’s genome, rich in biosynthetic gene clusters, could be a goldmine for biotechnology. Many of these clusters are involved in producing bioactive molecules similar to antibiotics and other useful compounds. If researchers can culture the organism in the lab and activate these pathways, it may lead to the discovery of entirely new classes of natural products.
What Scientists Still Need to Learn
Despite the groundbreaking findings, Ca. T. magnifica remains full of mysteries. The mechanism by which pepins form is still unknown. Do they originate from invaginations of the cell membrane, or do they assemble independently? Researchers also need to understand how the cell maintains genetic stability across so many copies of its genome.
Culturing this bacterium in laboratory conditions is another major challenge. So far, all observations have been made from specimens collected directly from the mangrove environment. Without a stable culture, scientists cannot yet study its full life cycle, environmental tolerance, or detailed biochemical functions.
Future research will focus on decoding how Ca. T. magnifica coordinates the activities of thousands of pepins, how it organizes its metabolism across centimeters of cytoplasm, and what evolutionary advantages this extraordinary structure provides.
A New Perspective on the Hidden World of Microbes
The discovery of Candidatus Thiomargarita magnifica is a powerful reminder that our understanding of microbial life is still incomplete. Even in habitats that have been studied for decades, such as coastal mangroves, life continues to surprise us.
This bacterium demonstrates that simplicity and complexity are not absolute categories. Instead, they exist on a spectrum shaped by evolutionary innovation. A single bacterial cell, visible without a microscope, has now blurred the boundary between the microscopic and the macroscopic, between the simple and the complex.
As scientists continue to explore Earth’s diverse ecosystems with modern imaging and sequencing tools, discoveries like Ca. T. magnifica remind us that life still has many secrets waiting to be uncovered. From the smallest microbes to the largest animals, the story of biology is far from complete.
The research was published in Science on June 23, 2022.
This article has been fact checked for accuracy, with information verified against reputable sources. Learn more about us and our editorial process.
Last reviewed on .
Article history
- Latest version
- Last updated by Dayyal Dungrela, MLT, BSc, BS
Reference(s)
- Volland, Jean-Marie., et al. “A centimeter-long bacterium with DNA contained in metabolically active, membrane-bound organelles.” Science, vol. 376, no. 6600, 23 Jun 2022, pp. 1453-1458., doi: 10.1126/science.abb3634. <https://www.science.org/doi/full/10.1126/science.abb3634>.
Cite this page:
- Posted by Tamseel Fatima