Female Infertility: Causes and Investigations
Female infertility is a condition characterized by the inability to conceive after 12 months of regular unprotected intercourse, or after 6 months if the woman is over 35. It affects millions globally, impacting individuals, families, and communities. Infertility can be attributed to female factors, male factors, or a combination of both in about two-thirds of cases, with the remaining cases having unknown causes. The primary symptom is the inability to become pregnant. Factors contributing to female infertility include abnormalities in the ovaries, uterus, fallopian tubes, and endocrine system. A menstrual cycle that’s too long (over 35 days), too short (less than 21 days), irregular, or absent may indicate a lack of ovulation, a common cause of infertility. Successful pregnancy requires each step of the human reproduction process to function correctly.
Female Reproductive System
The ovaries serve as the production sites for female gametes, also known as ova, through a process called oogenesis. These ova are cyclically released at regular intervals through ovulation. Each ovary houses a multitude of follicles, each containing ova at different developmental stages.
During each menstrual cycle, up to 20 primordial follicles are activated for maturation. However, only one follicle reaches full maturity. This dominant follicle then ruptures to release the secondary oocyte from the ovary. The maturation of the follicle is stimulated by the follicle-stimulating hormone (FSH), which is secreted by the anterior pituitary gland.
As the follicle matures, it secretes estrogen, leading to the proliferation of the endometrium of the uterus, known as the proliferative phase. The follicular cells also secrete inhibin, which regulates the release of FSH by the anterior pituitary. A decrease in FSH levels triggers the secretion of luteinizing hormone (LH) by the anterior pituitary, known as the LH surge. This surge causes the follicle to rupture, expelling the ovum into the peritoneal cavity near the fimbrial end of the fallopian tube.
The fallopian tubes serve as conduits for the ova, transporting them from the ovaries to the uterus. The fertilization of the ovum by the sperm typically occurs within the fallopian tube.
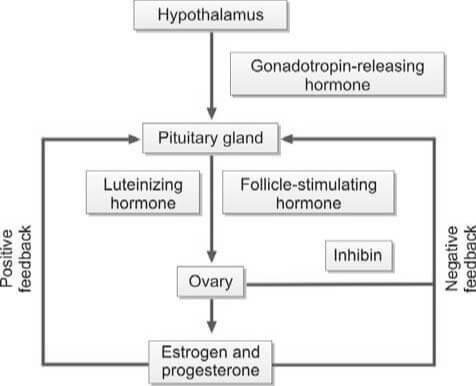
The ovum is composed of the secondary oocyte, the zona pellucida, and the corona radiata. When the follicle in the ovary ruptures, it collapses and fills with a blood clot, forming the corpus luteum. Luteinizing hormone (LH) transforms granulosa cells in the follicle into lutein cells, which begin to secrete progesterone.
Progesterone stimulates secretion from the endometrial glands, marking the secretory phase that was previously influenced by estrogen. As progesterone levels rise, LH production from the anterior pituitary is inhibited. Without LH, the corpus luteum regresses and becomes the non-functional corpus albicans. Following the regression of the corpus luteum, the production of estrogen and progesterone ceases, and the endometrium collapses, initiating menstruation.
If the ovum is fertilized and implants in the uterine wall, the developing placenta secretes human chorionic gonadotropin (hCG) into the maternal circulation. hCG maintains the corpus luteum for the secretion of estrogen and progesterone until the 12th week of pregnancy. After the 12th week, the corpus luteum regresses to the corpus albicans, and the placenta takes over the synthesis of estrogen and progesterone until childbirth.
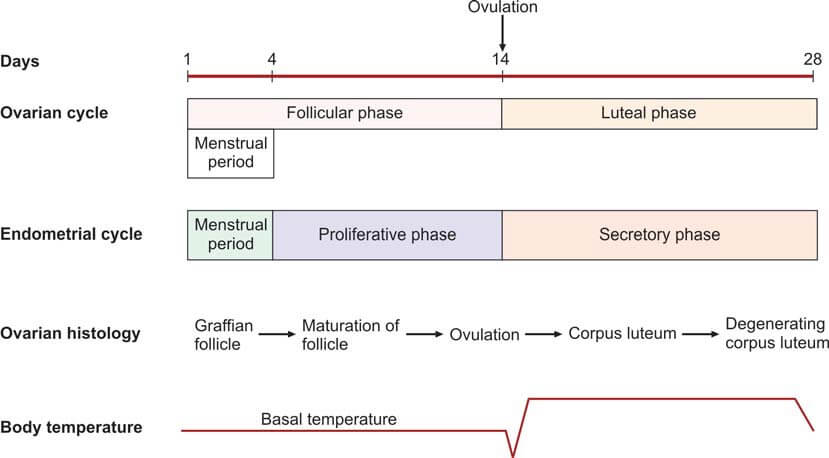
The average duration of a normal menstrual cycle is 28 days, with ovulation typically occurring around the 14th day of the cycle. The interval between ovulation and menstruation, known as the luteal phase, is fairly constant at 14 days.
Factors Contributing to Female Infertility
The following are the primary causes of female infertility:
- Dysfunction of the Hypothalamic-Pituitary Axis:
- Hypothalamic Factors:
- Overexertion through exercise
- High levels of stress
- Underweight conditions
- Kallman's syndrome
- Unexplained causes (Idiopathic)
- Pituitary Factors:
- Hyperprolactinemia
- Hypopituitarism (manifestations include Sheehan's syndrome, Simmond's disease)
- Craniopharyngioma
- Exposure to cerebral irradiation
- Hypothalamic Factors:
- Ovarian Dysfunction:
- Polycystic Ovarian Syndrome (Stein-Leventhal syndrome)
- Luteinized Unruptured Follicle
- Turner's syndrome
- Exposure to radiation or chemotherapy
- Surgical removal of ovaries
- Unexplained causes (Idiopathic)
- Impairment of the Reproductive Tract:
- Fallopian Tubes:
- Infections such as Tuberculosis, Gonorrhea, and Chlamydia
- History of surgical procedures (e.g., laparotomy)
- Tubectomy
- Congenital conditions like hypoplasia or non-canalization
- Endometriosis
- Uterus:
- Uterine malformations
- Asherman's syndrome
- Tuberculous endometritis
- Fibroids
- Cervix: Presence of sperm antibodies
- Vagina: Septum
- Fallopian Tubes:
- Sexual Dysfunction: Dyspareunia
Investigations
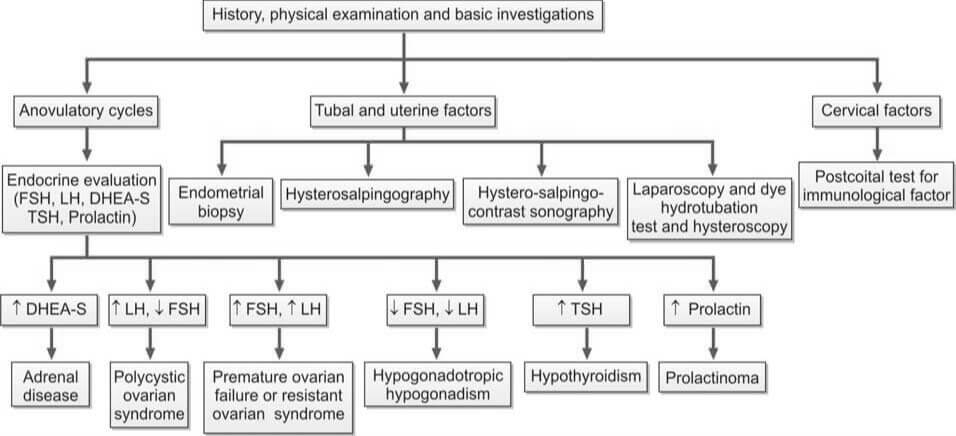
Evaluation of female infertility is shown in Flowchart 2.
Ovulation Testing Methods
Anovulation is the most prevalent cause of female infertility.
- Indicators of Ovulatory Cycles: Regular menstrual cycles, mastalgia, and the direct visualization of the corpus luteum through laparoscopy suggest ovulatory cycles. Anovulatory cycles are clinically manifested by amenorrhea, oligomenorrhea, or irregular menstruation. However, anovulation may still occur in seemingly regular cycles.
- Endometrial Biopsy: This procedure is performed during the premenstrual period (21st-23rd day of the cycle). The presence of a secretory endometrium during the latter half of the cycle serves as evidence of ovulation.
- Ultrasonography (USG): Serial ultrasonography begins from the 10th day of the cycle, measuring the size of the dominant follicle. A size greater than 18 mm suggests imminent ovulation. The collapse of the follicle and the presence of a few milliliters of fluid in the pouch of Douglas indicate ovulation. USG is also useful for treatment purposes (i.e., timing of coitus or intrauterine insemination) and diagnosing a luteinized unruptured follicle (absence of dominant follicle collapse). Transvaginal USG is more sensitive than abdominal USG.
- Basal Body Temperature (BBT): The patient measures her oral temperature at the same time every morning before getting up. A drop of about 0.5°F at the time of ovulation is observed. During the second (progestational) half of the cycle, the temperature slightly rises above the preovulatory level (increase of 0.5° to 1°F). This rise is due to the slight pyrogenic action of progesterone and thus serves as presumptive evidence of a functional corpus luteum.
- Cervical Mucus Study:
- Fern Test: During the estrogenic phase, a characteristic fern pattern appears when cervical mucus is spread on a glass slide. This ferning disappears after the 21st day of the cycle. If previously observed, its disappearance is presumptive evidence of corpus luteum activity.
- Spinnbarkeit Test: Cervical mucus is elastic and can stretch up to a distance of over 10 cm. This phenomenon, known as Spinnbarkeit or the thread test, indicates estrogen activity. During the secretory phase, the viscosity of the cervical mucus increases, and it fractures when stretched. This change in cervical mucus is evidence of ovulation.
- Vaginal Cytology: The Karyopyknotic Index (KI) is high during the estrogenic phase and decreases in the secretory phase. This index refers to the percentage of superficial squamous cells with pyknotic nuclei to all mature squamous cells in a lateral vaginal wall smear. Typically, a minimum of 300 cells are evaluated. The peak KI usually corresponds with the time of ovulation and may reach up to 50 to 85.
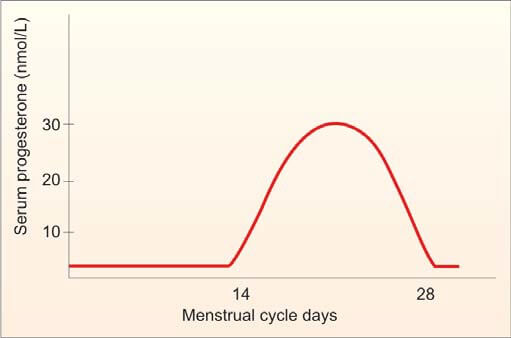
- Estimation of Progesterone in Mid-Luteal Phase (day 21 or 7 days before expected menstruation): A progesterone level greater than 10 nmol/L is a reliable indicator of ovulation if cycles are regular. An improperly timed sample is a common cause of abnormal results.

Diagnostic Procedures for Anovulation
The following tests are performed to identify the cause of anovulation:
- Measurement of LH, FSH, and Estradiol Levels (Days 2 to 6): In cases of hypogonadotropic hypogonadism, which is a result of hypothalamic or pituitary failure, all these values are typically low.
- Measurement of TSH, Prolactin, and Testosterone Levels (If Cycles are Irregular or Absent):
- Increased TSH Levels: This is indicative of hypothyroidism.
- Increased Prolactin Levels: This could suggest the presence of a pituitary adenoma.
- Increased Testosterone Levels: This could be a sign of Polycystic Ovarian Disease (PCOD) or Congenital Adrenal Hyperplasia. To differentiate between PCOD and Congenital Adrenal Hyperplasia, an ultrasound and estimation of dihydroepiandrosterone (DHEA) are performed.
- Transvaginal Ultrasonography: This procedure is conducted to detect the presence of PCOD.
Evaluating Tubal and Uterine Health
The following investigations are conducted to assess the status of the fallopian tubes and uterus:
- Infectious Disease Testing: This includes an endometrial biopsy for tuberculosis and a test for Chlamydial IgG antibodies to determine the tubal factor in infertility.
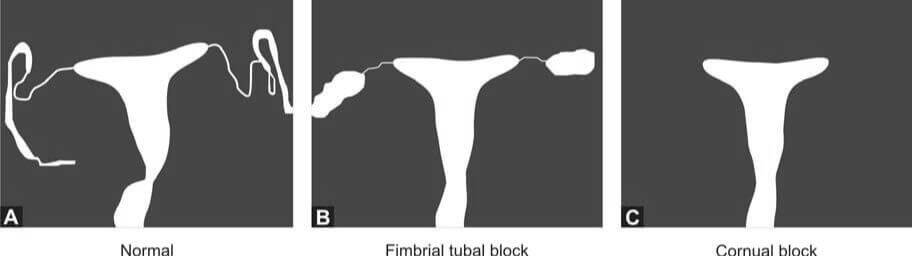
- Hysterosalpingography (HSG): HSG is a radiological contrast study used to examine the shape of the uterine cavity and to detect any blockage in the fallopian tubes. A catheter is inserted into the cervical canal, and a radiocontrast dye is injected into the uterine cavity. Real-time X-ray imaging is then performed to observe the flow of the dye into the uterine cavity, tubes, and its spillage into the uterine cavity.
- Hysterosalpingo-Contrast Sonography: In this procedure, a catheter is introduced into the cervical canal, and an echocontrast fluid is injected into the uterine cavity. The shape of the uterine cavity, the filling of the fallopian tubes, and the spillage of the contrast fluid are observed. Additionally, an ultrasound scan of the pelvis provides information about any existing fibroids or polycystic ovarian disease.
- Laparoscopy and Dye Hydrotubation Test with Hysteroscopy: This test involves inserting a cannula into the cervix and introducing methylene blue dye into the uterine cavity. If the tubes are patent, the dye will be observed spilling from the ends of both tubes. This technique also allows for the visualization of pelvic organs, endometriosis, and pelvic adhesions. If necessary, endometriosis and tubal blockage can be treated during the procedure.
Please note that potential pregnancy and active pelvic or vaginal infection are contraindications to tubal patency tests.
References
- Brugh III, VM, Lipshultz LI. Male factor infertility: Evaluation and management. Med Clin N Am 2004;88:367-85.
- Burtis CA, Ashwood ER. Tietz fundamentals of clinical chemistry. 5th Ed. Philadelphia: WB Saunders Company, 2001.
- Dohle GR, Jungwirth A, Colpi G, Papp G, Pomerol J, Hargreave TB. Guidelines on male infertility. European Association of Urology, 2006.
- Hirsh A. Male subfertility. BMJ 2003;327:669-72.
- Hamilton-Fairley D, Taylor A. Anovulation. BMJ 2003;327:546-9.
- Jarrow JP. Diagnostic approach to the infertile male patient. Endocrinol Metab Clin N Am 2007;36:297-311.
- Khalaf Y. Tubal subfertility. BMJ 2003;327:610-3.
- Kolettis PN. Evaluation of the subfertile man. Am Fam Physician 2003;67:2165-73.
- Williams C, Giannopoulos T, Sherriff EA. Investigation of infertility with the emphasis on laboratory testing and with reference to radiological imaging. J Clin Pathol 2003;56:261-7.
- Comment
- Posted by Dayyal Dg.